Evaluation of nematicidal potential of neem sawdust against Meloidogyne arenaria on eggplant
DOI:
https://doi.org/10.14719/pst.1485Abstract
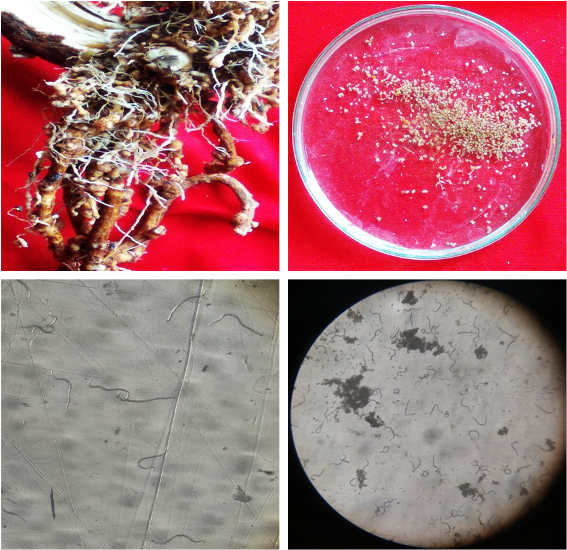
The present study was conducted to evaluate the impact of amending soil with decomposed neem (Azadirachta indica) sawdust at different concentrations (0-100%) against different inoculum levels (0-4000) of root-knot nematode (Meloidogyne arenaria) infecting the eggplants. Various physico-chemical properties of the soil increase as the concentration of decomposed neem sawdust (NSD) in the field soil increases. Nevertheless, the nitrogen content of the soil decreased with a progressive fluctuation in NSD. Lower levels (10-30%) of NSD, with and without different inoculum levels, improve the plant growth and photosynthetic pigment content significantly compared to controls (plants with uninoculated soil) as well as inoculated plants. The maximum improvement in the growth and photosynthetic pigments was recorded at 30% NSD soil amendment and this was continuously effective against all the nematode inoculum levels. At higher levels of NSD (40-100%), all the studied growth and photosynthetic parameters were decreased gradually to control and a similar reductional trend was also observed on nematode inoculated eggplants. On root-knot nematode reproduction, NSD at all levels progressively suppressed the number of egg masses but enhanced the number of galls only up to 30%. Galling was, however, totally absent in 70% and onward dust treatments of eggplants. Conclusively, NSD suppressed plant growth and photosynthetic pigments along with nematode buildup beyond 40% amendments. Thus, lower NSD levels (up to 30% amendments) will be recommended as growth and photosynthetic pigments supplement to eggplants, which also have nematicidal potential against egg masses of nematodes.
Downloads
References
Palomares-Rius JE, Escobar C, Cabrera J, Vovlas A, Castillo P. Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front. Plant Sci. 2017; 8:1987.doi: 10.3389/fpls. 01987. https://doi.org/10.3389/fpls.2017.01987
Abad P, Favery B, Rosso MN, Castagnone-Sereno P. Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003; 4:217–24. doi:10.1046/j.1364-3703.2003.00170.x. https://doi.org/10.1046/j.1364-3703.2003.00170.x
Vieira P and Gleason C. Plant-parasitic nematode effectors - insights into their diversity and new tools for their identification. Curr Opin Plant Biol. 2019; 50:37–43. doi: 10.1016/j.pbi.2019.02.007. https://doi.org/10.1016/j.pbi.2019.02.007
Navyashree B, Dharmashekar C, Shivamallu C, Balasubramanian S, Prasad SK, Prasad KS, Latha KC. Plant induced resistance in Solanacearum lycopersicum species against root-knot nematodes. J App Biol Biotech. 2021;9(1):88-95. doi:10.7324/JABB.2021.9112. https://doi.org/10.7324/JABB.2021.9112
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 2013;14:946–61. doi:10.1111/mpp.12057. https://doi.org/10.1111/mpp.12057
Li X, Xing X, Xu S, Zhang M, Wang Y, Wu H. Genome-wide identification and functional prediction of tobacco lncRNAs responsive to root-knot nematode stress. PLoS One. 2018; 13(11):e0204506.https://doi.org/10.1371/journal. pone.0204506
Bohlman H, Sobczak M. The plant cell wall in the feeding sites of cyst nematodes. Front Plant Sci. 2014; 5: 89. https://doi.org/10.3389/fpls.2014.00089
Otles S, Ozgoz S. Health effects of dietary fiber. Acta Sci Pol Technol Aliment. 2014;13 (2):191-202.doi:10.17306/J.AFS.2014.2.8. https://doi.org/10.17306/J.AFS.2014.2.8
Weese T and Bohs L. Eggplant origins: out of Africa, into the Orient. Taxon. 2010; 59:49–56.doi:10.2307/27757050. https://doi.org/10.1002/tax.591006
FAO. FAOSTAT. Production Databases. Available online at: http://www.faostat.fao.org (Accessed January 30, 2017)
Taher D, Solberg SO, Prohens J, Chou Y, Rakha M, Wu T. World vegetable center eggplant collection: Origin, composition, seed dissemination and utilization in breeding. Front Plant Sci. 2017; 8:1484.doi:10.3389/fpls.01484. https://doi.org/10.3389/fpls.2017.01484
Statista. Production volume of brinjal in India FY 2015-2020 with an estimate for 2021. Available online at:https://www.statista.com/statistics/1038975/india-production-of-eggplant/ statista research department. Oct 16, 2021
USDA ARS (United States Department of Agriculture Agricultural Research Service), National Nutrient Database for Standard Reference Release 28 (https://ndb.nal.usda.gov/ndb/).https://ndb.nal.usda.gov/ndb/foods/show/2962?fgcd=&manu=&lfacet=&format=&count=&max=50&offset=&sort=default&order=asc&qlookup=eggplant&ds=&qt=&qp=&qa=&q&q=&ing=/Accessed 14 March 2018.
Casati L, Pagani F, Braga PC, Scalzo RL, Sibilia V. Nasunin, a new player in the field of osteoblast protection against oxidative stress. J Funct Foods. 2016; 23:474–84. https://doi.org/10.1016/j.jff.2016.03.007
Koul O, Wahab S. Neem: Today and in the New Millennium. New York, NY: Kluwer Academic Publishers, Springer.1st ed. 2004 edition (23 January 2011).ISBN.13:978904816269.http://www.ebooks.kluweronline.com,http://www.springeronline.com
Chaudhary S, Kanwar RK, Sehgal A, Cahill DM, Barrow CJ, Sehgal R, Kanwar JR. Progress on Azadirachta indica Based Biopesticides in Replacing Synthetic Toxic Pesticides. Front Plant Sci. 2017; 8:610.doi:10.3389/fpls. 00610
Akhtar M. Nematicidal potential of the neem tree Azadirachta indica (A. Juss). Integr. Pest Manag. Rev. 2000; 5: 57–66.doi:10.1023/A:1009609931223
Loknadhan S, Muthukrishnan P, Jeyaraman S. Neem products and their agricultural applications. J Biopestic. 2012; 5 (Supplementary):72-76. http://www.jbiopest.com/users/lw8/efiles/vol_5_0_72_76f.pdf
Prakash J, Singh K. Control of root-knot nematode by using composted sawdust in tomato root. Afr J Biotechnol. 2014; 13:4070-80.doi:10.5897/AJB2014.13726
Mondal E, Chakraborty K. Azadirachta indica: A tree with multifaceted applications: An Overview. J Pharm Sci Res. 2016; Vol. 8 (5):299-306. https://www.jpsr.pharmainfo.in
Alhassanl YJ, Haruna Y, Muhammad MA, Firddausi SK. Economics of bio-based fertilizer in improving crop productivity through extension services delivery. Int J Agric Plant Sci. 2019; 1 (4):10-13. Online ISSN: 2664-7664; Print ISSN: 2664-7656. www.agriculturejournal.in
Siddiqui MA and Alam MM. Sawdust as soil amendments for control of nematodes infesting some vegetables. Biol. Wastes. 1990; 33:123-29. https://doi.org/10.1016/0269-7483(90)90152-I
Yadav S, Patil J, Kumar A. Bio-nematicidal effect of Azadirachta indica, against Meloidogyne incognita in tomato. In J Chem Stud. 2018;6(3):2757-61.PISSN:23498528.EISSN:23214902.https://www.chemijournal.com/archives/2018/vol6issue3/PartAN/6-3-244-235.pdf
Oka Y. Mechanism of nematode suppression by organic soil amendment: A review. Appl Soil Ecol. 2010; 44:101-15. https://doi.org/10.1016/j.apsoil.2009.11.003
Bernard GC, Egnin M, Bonsi C.The Impact of Plant-Parasitic Nematodes on Agriculture and Methods of Control. Nematology-Concepts, Diagnosis and Control. 2017; doi:10.5772/intechopen.68958
Sumbul A, Rizvi R, Salah M, Tiyagi SA, Ansari RA, Safiuddin, Mahmood I. Role of different sawdusts and bioinoculant in the management of root-knot nematode infesting chickpea. Asian J Crop Sci. 2015; 7: 197-206.doi:10.3923/ajcs.2015.197.206
Mahaney WC, Voros J, Krishnamani R, Hancock RGV, Aufreiter S, Milner M W, Allen CCR. Physicogeochemical and mineral composition of neem tree soils and relation to organic properties. Geografiska Annaler: Series A, Phys Geogr.2016;98:143–54.doi:10.1111/geoa.12129
Carter MR, Ball B. Soil porosity. In: Carter MR (Editor) Soil sampling and methods of analysis. Canadian Society of Soil Science. Lewis Publishers, CRC Press, Boca Raton. 1993;581–88. ISBN-13: 978-0-8493-3586-0 (alk. paper). http://www.crcpress.com
Black CA, Evans DD, White JL, Ensmingher LE, Clarke FE, Dinauer RC. Methods of soil analysis. Part I- Physical and mineralogical properties including statistics of measurement and sampling (9 Series Agronomy). Madison, WI: American Society of Agronomy. 1965.
Jackson ML. Soil chemistry analysis. Prentice Hall of India, Pvt. Ltd, 1st edition, New Delhi, India. 1973. 498
Ganguly AK. Base Exchange capacity of silica and silicate minerals. J Phys Chem. 1951;55:1417–28. http://dx.doi.org/10.1021/j150492a002.
Richard LA. Diagnasis and improvement of saline and alkali soils. Agric. Hand book, 60, U.S. Dept, Agric. Washington DC.1954:160. https://doi.org/10.2136/sssaj1954.03615995001800030032x
Kjeldahl J. A new method for the determination of nitrogen in organic matter. Zeitschrift für Analytische Chemie.1883; 22:366-82. http://dx.doi.org/10.1007/BF01338151
Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circ. United state Department of Agriculture. Circular 19, Washington DC.1954; 939:1-19.
Lindsay WL and Norvell WA. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci soc Am J. 1978; 42:421-28.doi:10.2136/sssaj1978.03615995004200030009x
Eisenback JD, Hunt DJ. General Morphology. In: Root Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., editors.; CABI: Wallingford, CT, USA, 2009; 18–54. ISBN9781845934927. doi:10.1079/9781845934927.0018
MacKinney G. Absorption of light of chlorophyll solutions. J Biol Chem. 1941; 140:315-22. https://doi.org/10.1016/S0021-9258(18)51320-X
Taylor AL and Sasser JN. Biology, Identification and control of root-knot nematodes (Meloidogyne spp.). Publ Dep Plant Pathol, North Carolina State Univ., and U.S. Agency Int. Dev. Raliegh, N.C. 1978 vii; 111.
Fisher RA. Statistical Methods for Research Workers. Oliver and Boyd, Edinburgh: Tweeddale court London: 33 Paternoster Row, E.C.1925.
Hassan MA, Chindo PS, Marley PS, Alegbejo. Management of root-knot nematodes (Meloidogyne spp.) on tomato (Lycopersicon lycopersicum) using organic wastes in Zaria, Nigeria. Plant Prot Sci. 2010; 46 (1):34-38. https://doi.org/10.17221/1/2009-PPS.
Yasin M, Jabran K, Afzal I, Iqbal S, Nawaz MA, Muhmood A, Asif M, Nadeem MA, Rahman ZU, Adnan M, Siddiqui M, Shahid MQ, Andreasen C. Industrial sawdust waste: An alternative to soilless substrate for garlic (Allium sativum L.). J Appl Res Med Aromat Plants. 2020; 18. 100252. doi: 10.1016/j.jmap.2020.100252
Seghatoleslami MJ, Mousavi G, Javedi H. Chicory (Cichorium intybus) responses to nitrogen and plant density in Birjand, Iran Int J Biosci. 2014; 4 (9):56-61. http://dx.doi.org/10.12692/ijb/4.9.56-61
Misganaw A, Alemay H, Kesete N. Review on the effect of level of nitrogen on growth and yield of onion in Ethiopia. J Glob Agric Ecol. 2021; 11 (1):1-7. Availablefrom:https://archives.biciconference.co.in/index.php/jogae/article/view/6272
Escobar C, Barcala M, Cabrera J, Fenoll C. Overview of root-knot nematodes and giant cells. Adv Bot Res. 2015; 73, 1–32.doi: 10.1016/bs.abr.2015.01.001
Bozbuga R. Characterisation of cell walls at the feeding site of Meloidogyne incognita, PhD thesis, University of Leeds, Leeds. 2017.
Favery B, Quentin M, Jaubert Possamai S, Abad P. Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J Insect Physiol. 2016; 84:60-69.doi:10.1016/j. jinsphys.2015.07.013
Wendimu GY. Biology, Taxonomy and Management of the Root-Knot Nematode (Meloidogyne incognita) in Sweet Potato, Adv Agric. 2021; vol. 2021:1-13 Article ID 8820211, 13 pages. https://doi.org/10.1155/2021/8820211.
Hossain MA, Al-Toubi WAS, Weli AM, Al-Riyami QA, Al Sabahi JN. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J Taibah Univ Sci. 2013;7:181–88. doi:10.1016/j.jtusci.2013.05.003
Hayat A, Javed N, Khan SA, Gondal AS, Khan HU. Effect of organic amendments on nematode galling index and egg masses production in potato inoculated with root-knot nematode. Pak J Phytopathol. 2012;24(1):01-05. http://pakps.com/pjp/files/01-05-hayat.pdf
Li J, Zou C, Xu J, Ji X, Niu X, Yang J, Huang X, Zhang KQ. Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol. 2015; 53:67-95. doi:10.1146/annurev-phyto-080614-120336. PMID: 25938277
Souza RAD, Ribeiro RCF, Rocha LDS, Xavier AA, Martins IPS, Silva FDJ. Reaction of Crambe abyssinica to Meloidogyne javanica and M. incognita race 3 Rev. Caatinga Mossoro. 2019; 32 (1):276-81. http://dx.doi.org/10.1590/1983-21252019v32n128rc
Debia PJG, Barros BCB, Puerari HH, Ferreira JCA , Dias-Arieira CR. Induced systemic resistance in beet plants infected with Meloidogyne javanica. Chil J Agric Res. 2021; 81 (1); 70-79. http://dx.doi.org/10.4067/S0718-58392021000100070
Akhtar SS, Li G, Andersen MN, Liu F. Biochar enhances yield and quality of tomato under reduced irrigation. Agricultural Water Management.2014; 138:37–44. https://doi.org/10.1016/j.agwat.
Kokalis - Burelle N, Rodriguez - Kabana R, Weaver CF, King PS. Evaluation of powdered pine bark for control of Meloidogyne arenaria and Heterodera glycines on soybean. Plant Soil. 1994;162:163-168.http://www.jstor.org/stable/42939537
Ohri P, Pannu SK. Effect of phenolic compounds on nematodes- A review. J Appl Nat Sci. 2010; 2 (2):344-50. doi https://doi.org/10.31018/jans.v2i2.144
Chitwood DJ, Perry RN. Reproduction, physiology and biochemistry. In: Root-Knot Nematodes (Perry, RN., Moens, M. and Starr, JL, eds), 2009; 182–200. Wallingford, Oxfordshire:CAB International Landaon
Thoden TC, Korthals GW, Termorshuizen AJ. Organic amendments and their influences on plant-parasitic and free-living nematodes: a promising method for nematode management ? Nematology, 2011; 13(2), 133-53. https://doi.org/10.1163/138855410X541834
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Asgar Ali, Kamal Singh

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









