Multifunctional attributes of endophytic Pseudomonas strains isolated from the leaves of medicinal plants
DOI:
https://doi.org/10.14719/pst.1597Keywords:
Aromatic degradation, Endophytic bacteria, IAA, Nitrogen fixation, PhytotoxicityAbstract
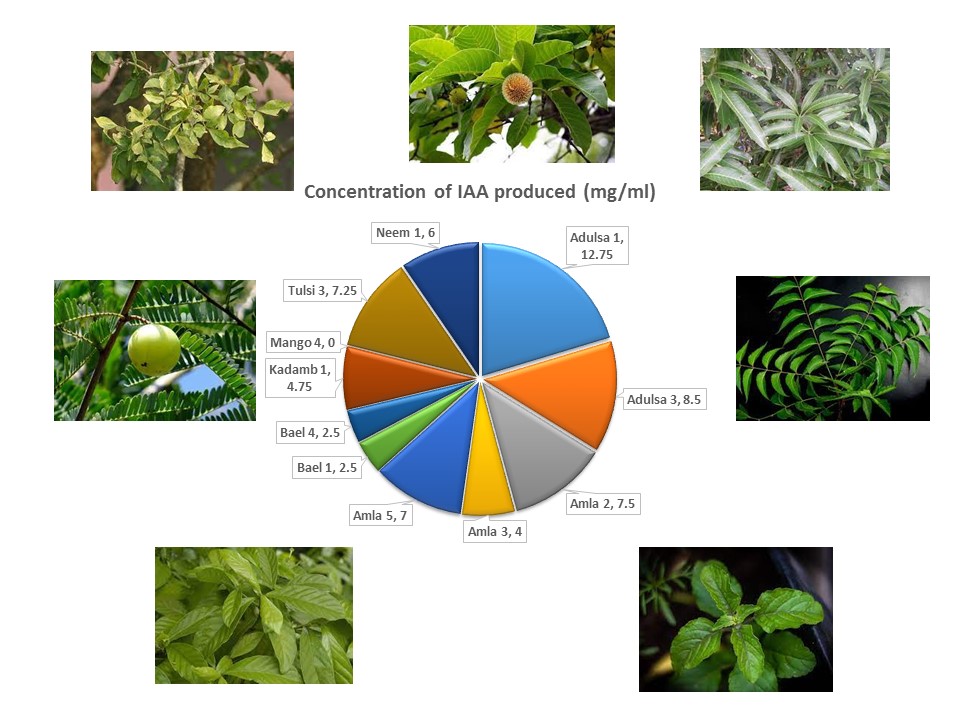
Endophytic bacteria are responsible for improved plant growth due to its role in nitrogen fixation, indole acetic acid (IAA) production, phosphate solubilization etc and in plant protection through various mechanisms and production of bioactive compounds. The purpose of this study was to determine the plant growth promoting potential of endophytic bacteria isolated from medicinal plants namely, Adulsa, Amla, Bael, Kadamb, Mango, Neem, Tulsi. Endophytic bacteria isolated from the medicinal plants, comprised of 68% Gram positive and 29% Gram negative bacteria. Seventeen distinctly unique Gram-negative endophytes were selected for further analysis. The selected endophytes were tentatively identified as Pseudomonas sp. The multifarious endophytes were capable of nitrogen fixation, phosphate solubilisation, indole acetic acid (IAA) production, production of antimicrobial compounds and aromatic compound degradation. Some of the endophytic strains were found to harbor plasmids that may play a role in aromatic compound degradation. This study emphasizes the potential of endophytic Pseudomonas species in enhancing plant growth and plant protection.
Downloads
References
Cocking EC. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil. 2003;252(1):169-75. doi: 10.1023/A:1024106605806.
Azevedo JL, Maccheroni Jr W, Pereira JO, De Araújo WL. Endophytic microorganisms a review on insect control and recent advances on tropical plants. Electron J Biotechnol. 2000;3(1):40-65. doi: 10.2225/vol3-issue1-fulltext-4.
Siciliano SD, Fortin N, Mihoc A, Wisse G, Labelle S, Beaumier D et al. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl Environ Microbiol. 2001;67(6):2469-75. doi: 10.1128/AEM.67.6.2469-2475.2001, PMID 11375152.
Nogueira EdM, Vinagre F, Masuda HP, Vargas C, Pádua VLMd, Silva Frd et al. Expression of sugarcane genes induced by inoculation with Glucanacetobacter diazotrophicus and Herbaspirillum rubrisubalbicans. Genet Mol Biol. 2001;24(1-4):199-206. doi: 10.1590/S1415-47572001000100027.
Lee KD, Bai Y, Smith D, Han HS, Supanjani. Isolation of plant-growth-promoting endophytic bacteria from Bean nodules. Res J Agric Biol Sci. 2005;1:232-36.
Lata H, Li XC, Silva B, Moraes RM, Halda-Alija L. Identification of IAA-producing endophytic bacteria from micropropagated Echinacea plants using 16S rRNA sequencing. Plant Cell Tissue Organ Cult. 2006;85(3):353-59. doi: 10.1007/s11240-006-9087-1.
Wakelin SA, Warren RA, Harvey PR, Ryder MH. Phosphate solubilisation by Penicillium spp. closely associated with wheat roots. Biol Fertil Soils. 2004;40(1):36-43. doi: 10.1007/s00374-004-0750-6.
James KD, Williams PA. Ntn genes determining the early steps in the divergent catabolism of 4- nitrotoluene and toluene in Pseudomonas sp. strain TW 3. J Bacteriol. 1998;180(8):2043-49. doi: 10.1128/JB.180.8.2043-2049.1998, PMID 9555884.
Costa JM, Loper JE. Characterization of siderophore production by the biological control agent Enterobacter cloacae. Mol Plant Microbe Interact. 1994;7(4):440-48. doi: 10.1094/MPMI-7-0440.
Rodelas B, Salmerón V, Martinez-Toledo MV, González-López J. Production of vitamins by Azospirillum brazilense in chemically – defined media. Plant Soil. 1993;153(1):97-101. doi: 10.1007/BF00010548.
Compant S, Clement C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010;42: 669-78. doi: 10.1016/j.soilbio.2009.11.024
Lata R, Chowdhury S, Gond SK, White JF Jr. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol. 2018;66(4):268-76. doi: 10.1111/lam.12855. Epub 2018 Feb 26. PMID: 29359344.
Verma H, Kumar D, Kumar V, Kumari M, Singh SK, Sharma VK et al. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms. 2021;9,1729. https://doi.org/10.3390/microorganisms9081729
Prasetyawan S, Sulistyowati L, Aulani A. Glucanase and chitinase from some isolates of endophytic fungus Trichoderma spp. IOP Conference Series: Materials Science and Engineering. 2018;299. 012026. 10.1088/1757-899X/299/1/012026. 55 https://doi.org/10.1088/1757-899X/299/1/012026
Li HM, Sullivan R, Moy M, Kobayashi DY, Belanger FC. Expression of a novel chitinase by the fungal endophyte in Poa ampla. Mycologia. 2004;96:526-36. https://doi.org/10.1080/15572536.2005.11832951
Gupta S, Pandey S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) Plants Front Microbiol. 2019;10:1506. doi: 10.3389/fmicb.2019.01506
Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169, 30-39. doi: 10.1016/j.micres.2013.09.009
Duijff BJ, Gianinazzi-Pearson V, Lemanceau P. Involvement of the outer membrane lipopolysaccharides in the endophytic colonization of tomato roots by biocontrol Pseudomonas fluorescens strain WCS417r. New Phytol. 1997;135(2):325-34. doi: 10.1046/j.1469-8137.1997.00646.x.
M’Piga P, Bélanger RR, Paulitz TC, Benhamou N. Increased resistance to Fusarium oxysporum f. sp. radicis – licopersici in tomato plants treated with the endophytic bacterium Pseudomonas fluorescens strain 63-28. Physiol Mol Plant Pathol. 1997;50(5):301-20. doi: 10.1006/pmpp.1997.0088.
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43(10):895-914. doi: 10.1139/m97-131.
Sharma VK, Nowak J. Enhancement of Verticillium wilt resistance in tomato transplants by in vitro coculture of seedlings with a plant growth promoting rhizobacterium (Pseudomonas sp. strain Ps JN). Can J Microbiol. 1998;44(6):528-36. doi: 10.1139/w98-017.
Rabiey M, Hailey LE, Roy SR et al. Endophytes vs tree pathogens and pests: can they be used as biological control agents to improve tree health? Eur J Plant Pathol. 2019; 155: 711-29. https://doi.org/10.1007/s10658-019-01814-y
Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep. 2001; 18: 448-59. https://doi.org/10.1039/b100918o
Berg G, Hallmann J. Control of plant pathogenic fungi with bacterial endophytes. Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes, Springer-Verlag, Berlin. 2006; p. 53-69. https://doi.org/10.1007/3-540-33526-9_4
Kloepper JW, Ryu CM. Bacterial endophytes as elicitors of induced systemic resistance. Schulz BJE, Boyle CJC, Sieber TN editors. Microbial root endophytes, Springer-Verlag, Berlin. 2006; p. 33-52. https://doi.org/10.1007/3-540-33526-9_3
Stierle A, Strobel GA, Stierle D. Taxol and taxene production by Taxomyces andreanae an endophytic fungus of pacific yew. Science. 1993;260:214-16. doi:10.1126/science. 8097061
Strobel GA. Endophytes as sources of bioactive products. Microbes Infect. 2003;5:535–544. doi:10.1016/S1286-4579(03)00073-x
Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. J Nat Prod. 2004;67:257-68. doi:10.1021/np030397v
Li J-Y, Strobel GA. Jesterone and hydroxy-jesterone antioomycete cyclohexanone epoxides from the endophytic fungi Pestalotiopsis jesteri. Phytochemistry. 2001;57:261-65. https://doi.org/10.1016/S0031-9422(01)00021-8
Li JY, Harper JK, Grant DM et al. Ambuic acid, a highly functionalized cyclohexenone with antifungal activity from Pestalotiopsis spp. and Monochaetia sp. Phytochemistry 2001;56:463-68. https://doi.org/10.1016/S0031-9422(00)00408-8
Daisy B, Strobel G, Ezra D, Hess W. Muscodor vitigenus sp. nov., an endophyte from Paullinia paullinioides. Mycotaxon. 2002;84:39-50.
Castillo U, Harper JK, Strobel GA et al. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol Lett. 2003;224:183-90. https://doi.org/10.1016/S0378-1097(03)00426-9
Castillo UF, Strobel GA, Ford EJ et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology. 2002;148:2675-85. https://doi.org/10.1099/00221287-148-9-2675
Porteous-Moore F, Barac T, Borremans B, Oeyen L, Vangronsveld J, van der Lelie D, Campbell CD, Moore ERB. Endophytic bacterial diversity in poplar trees growing on a BTEX- contaminated site: the characterization of isolates with potential to enhance phytoremediation. Syst Appl Microbiol. 2006;29(7):539-56. doi: 10.1016/j.syapm.2005.11.012.
Germaine KJ, Liu X, Cabellos GG, Hogan JP, Ryan D, Dowling DN. Bacterial endophyte enhanced phyto-remediation of the organochlorine herbicide 2, 4-dichlorophenoxyacetic acid. FEMS Microbiol Ecol. 2006;57(2):302-10. doi: 10.1111/j.1574-6941.2006.00121.x, PMID 16867147.
Berde CV, Bhosale PP, Chaphalkar SR. Plasmids of endophytic bacteria as vectors for transformation in plants. Int J Integr Biol. 2010;9:113-18.
Bhowmik B, Singh RP, Jayaraman J, Verma JP. Population dynamics of cotton endophytic Pseudomonas, their antagonism and protective action against major pathogens of cotton. Indian Phytopathol. 2002;55:124-32.
Zinniel DK, Lambrecht P, Harris NB, Feng Z, Kuczmarski D, Higley P et al. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol. 2003;68:2198-08. https://doi.org/10.1128/AEM.68.5.2198-2208.2002
Palleroni NJ. Pseudomonadaceae. In: Krieg NR, Holt JG, editors. Bergey’s manual of systematic bacteriology. USA: Williams & Wilkins; 1984. p. 140-219.
Qin S, Zhang YJ, Yuan B, Xu YP, Xing K, Wang J et al. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014;374:753-66. doi: 10.1007/s11104-013-1918-3
Yan X, Wang Z, Mei Y, Wang L, Wang X, Xu Q et al. Isolation, diversity and growth-promoting activities of endophytic bacteria from tea cultivars of Zijuan and Yunkang-10. Front Microbiol. 2018;9:1848. doi: 10.3389/fmicb.2018.01848
Berde CV, Berde VB, Kulkarni AS. Studies on phosphate solubilising bacteria from Sakhartar creek, Ratnagiri. Int J Inst Pharm Lif Sci. 2014;4(3):1-7.
Torres-Rubio MG, Valencia-Plata SA, Bernal-Castillo J, Martinez-Nieto P. Isolation of enterobacteria, Azotobacter sp. and Pseudomonas sp. producers of indole-3-acetic acid and siderophores, from Columbia rice rhizosphere. Rev Latinoam Microbiol. 2000;42:171-76.
Pal A, Paul A. In vitro antimicrobial activity screening of bacteria endophytic to ethnomedicinal plant Rauvolfia serpentina (L.) Benth. ex. Kurz. J Appl Biotechnol Rep. 2020;7(3):176-84. doi: 10.30491/jabr.2020.117881
Basu A, Dixit SS, Phale PS. Metabolism of benzyl alcohol via catechol ortho pathway in methylnaphthalene-degrading Pseudomonas putida CVS86. Appl Microbiol Biotechnol. 2003;62:579-85. https://doi.org/10.1007/s00253-003-1305-8
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Harbor Press Laboratory Press; 1989.
Zinniel DK, Lambrecht P, Harris NB, Feng Z, Kuczmarski D, Higley P et al. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol. 2002;68(5):2198-208. doi: 10.1128/AEM.68.5.2198-2208.2002, PMID 11976089.
Prasad MP, Dagar S. Identification and characterization of endophytic bacteria from fruits like Avacado and Black grapes. Int J Curr Microbiol. App. Sci. 2014;3(8):937-47.
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278(1):1-9. doi: 10.1111/j.1574-6968.2007.00918.x, PMID 18034833.
Penyalver R, García A, Ferrer A, Bertolini E, López MM. Detection of Pseudomonas savastanoi pv. savastanoi in olive plants by enrichment and PCR. Appl Environ Microbiol. 2000;66(6):2673-77. doi: 10.1128/AEM.66.6.2673-2677.2000, PMID 10831456.
Uppala S. Potentiality of endophytic microorganisms in the management of leaf blight disease of Amaranth. M.Sc. (Thesis). Kerala: Kerala Agricultural University; 2007. doi:10.13140/RG.2.2.33389.90083
Aravind R, Kumar A, Eapen SJ, Ramana KV. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett Appl Microbiol. 2009;48(1):58-64. https://doi.org/10.1111/j.1472-765X.2008.02486.x
Ramesh R, Joshi AA, Ghanekar MP. Pseudomonads: major antagonistic endophytic bacteria to suppress bacterial wilt pathogen, Ralstonia solanacearum in the egg plant (Solanum melongena L.) World J Microbiol Biotechnol. 2009;5(1):47-55.https://doi.org/10.1007/s11274-008-9859-3
Muthukumar A, Nakkeeran S, Eswaran A, Sangeetha G. In vitro efficacy of bacterial endophytes against the chilli damping-off pathogen Pythium aphanidermatum. Phytopathol Mediterr. 2010;49(2):179-86.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870-74. https://doi.org/10.1093/molbev/msw054
Uma Maheshwari T, Anbukkarasi K, Hemalatha T, Chendrayan K. Studies on phytohormone producing ability of indigenous endophytic bacteria isolated from topical legume crops. Int J Curr Microbiol App Sci. 2013;2(6):127-36.
Lins MRCR, Fontes JM, Vasconcelos NM, Santos DMS, Ferreira OE, Azevedo JC, Araujo JM, Lima GMS. Plant growth-promoting potential of endophytic bacteria isolated from cashew leaves. Afr J Biotechnol. 2014;13(33):3360-65. https://doi.org/10.5897/AJB2014.13835
Germaine K, Keogh E, Garcia-Cabellos G, Borremans B, Lelie D, Barac T et al. Colonisation of poplar trees by gfp expressing bacterial endophytes. FEMS Microbiol Ecol. 2004;48:109-18. doi: 10.1016/j.femsec.2003.12.009.
Reinhold-Hurek B, Hurek T. Life in grasses: diazotrophic endophytes. Trends Microbiol. 1998;6(4):139-44. doi: 10.1016/s0966-842x(98)01229-3, PMID 9587190.
Zakria M, Njoloma J, Saeki Y, Akao S. Colonization and nitrogen-fixing ability of Herbaspirillum sp. Strain B501 gfp1 and assessment of its growth-promoting ability in cultivated rice. Microbes Environ. 2007;22(3):197-206. doi: 10.1264/jsme2.22.197.
Elbeltagy A, Nishioka K, Sato T, Suzuki H, Ye B, Hamada T et al. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl Environ Microbiol. 2001;67(11):5285-93. doi: 10.1128/AEM.67.11.5285-5293.2001, PMID 11679357.
Oteino N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, Germaine KJ et al. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015;6:745. doi: 10.3389/ fmicb.2015.00745
Varga T, Hixson KK, Ahkami AH, Sher AW, Barnes ME, Chu RK et al. Endophyte-promoted phosphorus solubilization in Populus. Front Plant Sci. 2020;11:567918. doi: 10.3389/fpls.2020.567918
Rani L, Pandey RK, Kumar V. Isolation and screening of P-solubilising endophytic diazotropic bacteria from ethno-medicinal indigenous rice of Jharkhand. Int J Biotechnol Res. 2018;5(1):001-010.
Chen J, Zhao G, Wei Y, Dong Y, Hou L, Jiao R. Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth promoting effect on Chinese fir seedlings. Sci Rep. 2021;11:9081. https://doi.org/10.1038/s41598-021-88635-4
Sessitsch A, Reiter B, Berg G. Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Can J Microbiol. 2004;50(4):239-49. doi: 10.1139/w03-118, PMID 15213748.
Taiz L, Zeiger E. Plant physiology. 2nd ed. Sunderland, MA: Sinauer Associates, Inc; 1998.
Chen C, Bauske EM, Musson G, Rodriguez-Kabana R, Kloepper JW. Biological control of Fusarium wilt on cotton by use of endophytic bacteria. Biol Control. 1995;5(1):83-91. doi: 10.1006/bcon.1995.1009.
An C, Ma S, Shi X, Xue W, Liu C, Ding H. Diversity and antimicrobial activity of endophytic fungi isolated from Chloranthus japonicus Sieb in Qinling Mountains, China. Int J Mol Sci. 2020;21: 5958; doi:10.3390/ijms21175958
Deshmukh SK, Verekar SA, Bhave SV. Endophytic fungi: a reservoir of antibacterials. Front Microbiol. 2014;5:715. doi:10.3389/fmicb.2014.00715
Jayatilake PL, Munasinghe H. Antimicrobial activity of cultivable endophytic and rhizosphere fungi associated with “mile-a-minute,” Mikania cordata (Asteraceae). BioMed Res Int. 2020;5292571:7 pages. https://doi.org/10.1155/2020/5292571
Mohamad OAA, Li L, Ma J-B, Hatab S, Xu L, Guo J-W, Rasulov BA, Liu Y - H, Hedlund BP and Li W-J. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant Licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front Microbiol. 2018;9:924. doi: 10.3389/fmicb.2018.00924.
Santos IP, Silva LCN, Silva MV, Araújo JM, Cavalcanti MS, Lima VLM. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae). Front Microbiol. 20156:350. doi: 10.3389/fmicb.2015.00350.
Du W, Yao Z, Li J, Sun C, Xia J, Wang B, Shi D, Ren L. Diversity and antimicrobial activity of endophytic fungi isolated from Securinega su_ruticosa in the Yellow River Delta. PLoS ONE 2020; 15: e0229589. https://doi.org/10.1371/journal.pone.0229589
Doucete WJ, et al. Phytoremediation of dissolved phase trichloroethylene using mature vegetation. In: Wickraamanayakee GB, Hinchee RE, editors. Bioremediation and phytoremediation: chlorinated and recalcitrant compounds. USA: Battelle Press; 1998; p.251-56.
Barac T, Taghavi S, Borremans B, Provoost A, Oeyen L, Colpaert JV et al. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat Biotechnol. 2004;22(5):583-88. doi: 10.1038/nbt960, PMID 15077119.
Bhat MA, Tsuda M, Horiike K, Nozaki M, Vaidyanathan CS, Nakazawa T. Identification and characterization of a new plasmid carrying genes for degradation of 2,4-dichlorophenoxyacetate from Pseudomonas cepacia CSV90. Appl Environ Microbiol. 1994;60(1):307-12. doi: 10.1128/aem.60.1.307-312.1994, PMID 7509586.
Taghavi S, Barac T, Greenberg B, Borremans B, Vangronsveld J, van der Lelie D. Horizontal gene transfer to endogenous endophytic bacteria from poplar improved phyto-remediation of toluene. Appl Environ Microbiol. 2005;71(12):8500-05. doi: 10.1128/AEM.71.12.8500-8505.2005, PMID 16332840.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Chanda V Berde, Vikrant B Berde

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









