Optimization of physico-chemical parameters for the production of phycobilin protein blue pigment, phycocyanin from the cyanobacterial strain Pseudanabaena limnetica (Lemmermann) Komarek
DOI:
https://doi.org/10.14719/pst.2100Keywords:
Phycocyanin pigment, Pseudanabaena limnetica , Physico-chemical optimization, Cyanobacteria strain, Photo bioreactor systemsAbstract
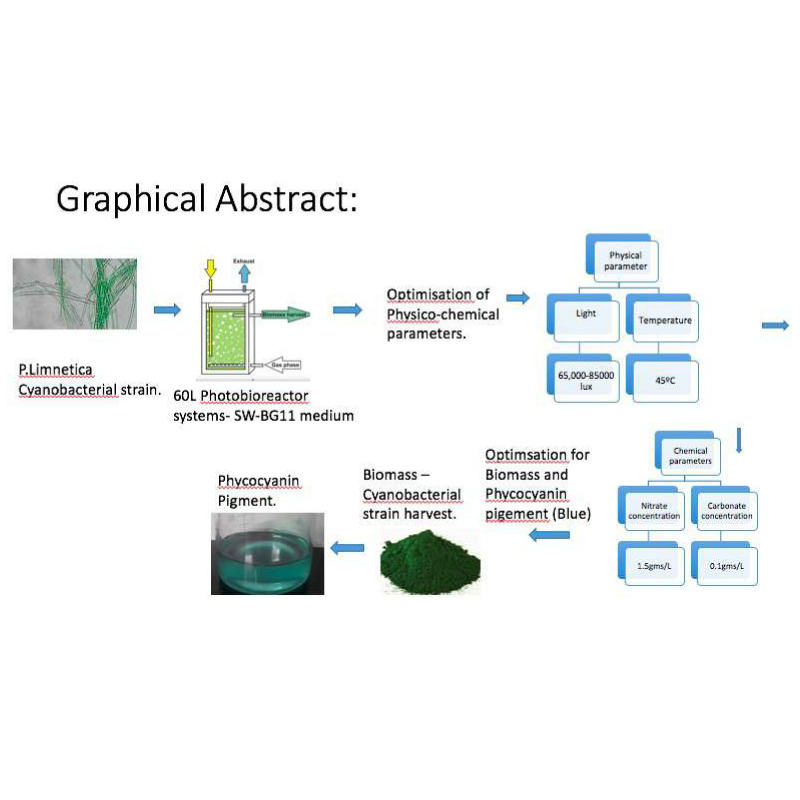
Pseudanabaena limnetica, the cyanophycean microalga, like other members of Cyanophycea, is an excellent source of pigments such as phycocyanin, proteins, carotenoids and polysaccharides. These strains also form a large proportion of algal biomass. The P.limnetica strain can grow in the extreme environmental conditions and it grows well in SW-BG 11 medium under laboratory conditions. In the present investigation, this cyanobacteria strain was isolated from the salt pans of Mulund Mumbai areas and it was cultivated in the lab under controlled conditions of light and temperature with optimum parameters of nitrate and carbonate concentrations. The culture was cultivated in the 60L photo bioreactor systems with the (65,000-85,000 lux) at 45?C in the SW-BG11 medium. The optimization experiments were carried out at the indoor and outdoor conditions. The nitrate and carbonate concentrations were optimized for obtaining maximum amount of algal biomass along with the blue-green phycocyanin pigment. The phycocyanin pigment was lyophilized for its further incorporation into the food and cosmetics products. The spectroscopic calculations of phycocyanin, allophycocyanin and phycoerythrin was done at 620, 650 and 562 nm using the Bennett and Bogorad equation. From the results obtained, it was concluded that 0.1gms/L and 1.5gms/L of the carbonate and nitrate concentrations, respectively, were the ideal concentrations for the further experiments for the cost effective production of P. limnetica in the SW-BG 11 medium. The outdoor conditions were found to be favorable for obtaining the maximum biomass and phycocyanin pigment production which would - make it more cost effective, commercially.
Downloads
References
Chen T, Wong Y, Zheng W. Purification and characterization of selenium-containing phycocyanin from selenium-enriched Spirulina platensis. Phytochemistry 2006; 67:2424-30.https://doi.org/10.1016/j.phytochem.2006.08.004
Jiang L, Wang Y, Liu G, Liu H, Zhu F, Ji H, Li B. C-Phycocyanin exerts anti-cancer effects via the MAPK signaling pathway in MDA-MB-231 cells. Cancer Cell Int. 2018 Jan 25;18:12. https://doi.org/10.1186/s12935-018-0511-5
18,12.10.1186/s 12935-018-0511-5.
Grover P, Bhatnagar A, Kumari N, Narayan Bhatt A, Kumar Nishad D, Purkayastha J. C-Phycocyanin-a novel protein from Spirulina platensis- In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J Biol Sci. 2021 Mar; 28(3):1853-1859. https://doi.org/ 10.1016/j.sjbs.2020.12.037.
Bechelli J, Coppage M, Rosell K, Liesveld J, Cytotoxicity of Algae Extracts on Normal and Malignant Cells, Leukaemia Research and Treatment, vol. 2011, Article ID 373519, 7 pages, 2011. https://doi.org/10.4061/2011/373519
Eriksen NT. Production of phycocyanin--a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol. 2008 Aug;80(1):1-14. https://doi.org/10.1007/s00253-008-1542-y.
Mogany T, Kumari S, Swalaha F.M, Bux F, Extraction and characterisation of analytical grade c-phycocyanin from Euhalothece sp., Journal of Applied Phycology 2019 Vol.31 No.3,pp.1661-1674 https://doi.org/10.1007/s10811-018-1661-5.
Carlozzi P, Dilution of solar radiation through “culture” lamination in photobioreactor rows facing south-north: a way to improve the efficiency of light utilization by cyanobacteria (Arthrospira platensis), Biotechnology and Bioengineering, (2003), vol. 81, no. 3, pp. 305–315. https://doi.org/10.1002/bit.10478
Vonshak A, Role of light and photosynthesis on the acclimation process of the cyanobacteria Spirulina platensis to salinity stress. J Appl Phycol, (1996), 8:119. Pp. 119-124. https://doi.org/10.1007/BF02186314
Pagels F, Guedes AC, Amaro HM, Kijjoa A, Vasconcelos V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol Adv. 2019 May-Jun;37(3):422-443. https://doi.org/10.1016/j.biotechadv.2019.02.010.
Schipper K, Production of Phycocyanin by Leptolyngbya sp. in desert environments., AlgalResearch,(2020), 47. https://doi.org/10.1016/j.algal.2020.101875
Magar C, Deodhar M, Operational Strategies for Cost Effective Mass Cultivation of Halophilic Microalgal Strain Pseudanabaena limnetica in 1000 L Flat Panel Photobioreactor, J Pet Environ Biotechnol, (2018) 9:4. https://doi.org/10.4172/2157-7463.1000380
Bennett A, Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419-35. https://doi.org/10.1083/jcb.58.2.419.
Safaei M, Maleki H, Soleimanpour H, Norouzy A, Zahiri HS, Vali H, Noghabi KA. Development of a novel method for the purification of C-phycocyanin pigment from a local cyanobacterial strain Limnothrix sp. NS01 and evaluation of its anticancer properties. Sci Rep. 2019 Jul 1;9(1):9474. https://doi.org/10.1038/s41598-019-45905-6.
Shukla SP, Antarctic cyanobacteria as a source of phycocyanin: An assessment, Indian journal of Geo-Marine sciences, (2008), 37(4), pp: 446-449.
Kenekar A, Deodhar M, Effect of varying physico-chemical parameters for the production of phycobiliprotein of indigenous isolate Geitlernema sulphureum, (2013), Vol 12, Issue 3, pp: 146-154. https://doi.org/10.3923/biotech.2013.146.154 Sharma G, Kumar M, Ali MI, Jasiya ND, Effect of carbon content, salinity and pH on Spirulina Plantensis for Phycocyanin, Allophycocyanin and Phycoerythrin Accumalation, J Microb Biochem Technol, (2014), 6:202-206. https://doi.org/10.4172/1948-5948.1000144
Deshmukh D, Statistical evaluation of nutritional components impacting phycocyanin production in Synechocystis spp, Brazilian Journal of Microbiology, (2012), 348-355. https://doi.org/10.1590/S1517-838220120001000041
Rambhiya S, Magar C, Deodhar M. Using seawater-based Na2CO3 medium for scrubbing the CO2 released from Bio-CNG plant for enhanced biomass production of Pseudanabaena limnetica Applied sciences, (2021), Volume 3, Article no: 276. https://doi.org/10.1007/s42452-021-04271-7
Castro GFP. Biomass production by Arthrospira platensis under different culture conditions. Food Sci. Technol. (2015) 35, 18–24. https://doi.org/10.1590/1678-457X.6421
Downloads
Published
Versions
- 01-04-2023 (2)
- 08-02-2023 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Ashwini Tribhuvan, Manjushri Deodhar , Ajit Kengar

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









