Potentials of kawayang tinik (Bambusa blumeana) as new source antimicrobial agents
DOI:
https://doi.org/10.14719/pst.1451Keywords:
Antimicrobial, Bambusa blumeana, BambooAbstract
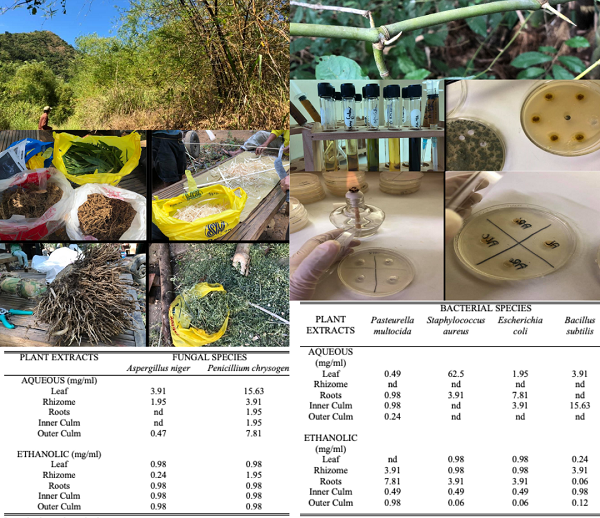
In this time where health is priority and surges of microbial resistance is highly observed within the society, discovering new, effective and sustainable sources of potential pharmacologic products is highly significant. The study explored the antimicrobial potentials of the different parts of Bambusa blumeana (kawayang tinik), a common Philippine bamboo species, against selected pathogenic bacterial and fungal species utilizing minimum inhibitory concentration via agar well diffusion method. Results of the study showed that extracts of B. blumeana, specifically the leaf, rhizome, roots, inner and outer culms, are capable of inhibiting microbial growth at 0.06 to 0.98 mg/ml concentrations. Specifically, the aqueous outer culm extract of B. blumeana proved to be most effective in inhibiting the growth of Pasteurella multocida at 0.24 mg/ml while Staphylococcus aureus and Escherichia coli were most susceptible to ethanolic outer culm extracts at 0.06 mg/ml and 0.12 mg/ml respectively. Bacillus subtilis, on the other hand, was observed to be the most sensitive to ethanolic root extracts at 0.06 mg/ml. Furthermore, Aspergillus niger was observed to be susceptible to ethanolic rhizome extract (0.24 mg/ml) while the ethanolic leaf, roots, inner and outer culms were equally effective in inhibiting Penicillium chrysogenum at 0.98 mg/ml extract concentration. Phytochemical testing further revealed the presence of phenols and flavonoids in the different parts of the bamboo species which further support its potential as a new source of pharmaceutical biocompounds.
Downloads
References
Tantengco OAG, Condes MLC, Estadilla HHT, Ragragio EM. Ethnobotanical survey of medicinal plants used by Ayta communities in Dinalupihan, Bataan, Philippines. Pharmacogn J. 2018;10(5):859-70. https://doi.org/10.5530/pj.2018.5.145
Kalita C, Ganguly M, Devi A. Evaluation of antioxidant capacity and antimicrobial properties of ethnic Bambuseae species and identification of the active components. International Journal of Pharmaceutical and Biological Achieves. 2016;3(1):61-71.
Nguyen PT, Xuan TD, Ha PT, Tu anh TT, Khanh TD. Inhibitory effects of bamboo leaf on the growth of Pyricularia grisea fungus. Molecular Diversity Preservation International: Agriculture [Internet]. 2018 [cited 2018 Nov 30];8(92):1-8. https://doi.org/10.3390/agriculture8070092
Mahunu GK, Zhang H, Tibiru M, Qiya A, Xiaoyun Y, Zhao ZL. Bamboo leaf flavonoid enhances the control effect of Pichia caribbica against Penicillium expansum growth and Patulin accumulation in apples. Postharvest Biology and Technology [Internet]. 2018 [cited 30 June2019];141:1-7. https://doi.org/10.1016/j.postharvbio.2018.03.005
Wasnik DD, Tumane PM. Antibacterial activity of Bambusa bambose L. against multiple drug resistant (MDR) bacteria isolated from clinical specimen. International Journal of Pharmaceutical Sciences. 2014;25(1):215-18.
Ambika K, Rajagopal B. In vitro antimicrobial and antiproliferative activity of Bambusa vulgaris. International Journal of Pharmacy and Pharmaceutical Research. 2017;9(1):10-22.
Chuah EL, Zakaria ZA, Suhaili Z, Abu bakar S and Desa MNM. Antimicrobial activities of plant extracts against Methicillin – Susceptible and Methicillin – Resistant Staphylococcus aureus. Journal of Microbiology Research [Internet]. 2014 [cited 30 June 2018]; 4(1):6-13. https://doi.org/10.5923/j.microbiology.20140401.02
Tanaka A, Zhu Q, Tan H, Horiba H, Ohnuki K, Mori Y et al. Biological activities and phytochemical profiles of extracts from different parts of Bamboo (Phyllostachys pubescens). Molecules [Internet]. 2014 [cited 30 June 2018];1(19):8238-60. https://doi.org/10.3390/molecules19068238
Selvamohan T, Ramadas V, Shibila S, Kishore S. Antimicrobial activity of selected medicinal plants against some selected human pathogenic bacteria. Pelagia Research Library Advances in Applied Science Research. 2012;3(5):3374-81.
Sule IO, Agbabiaka TO. Antibacterial effect of some plant extracts on selected Enterobacteriaceae. Ethnobotanical Leaflets. 2008;1(2):1035-42.
Lakshmanan K, Arumungam A, Mani R. Phytochemical screening and in – vitro antimicrobial activity of Vitex negundo L var. purpurascens Sivar and Mold Against Pathogenic Microorganisms. Drug Invention Today. 2012;4(12):667.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. Journal of Pharmaceutical Analysis. 2016;(6):71-79. https://doi.org/10.1016/j.jpha.2015.11.005
Islam MA, Alam MM, Choudhury ME, Kobayashi N, Ahmed MU. Determination of minimum inhibitory concentration (MIC) of cloxacillin for selected isolates of Methicillin- Resistant Staphylococcus aureus (MRSA) with their antibiogram. Bangladesh Journal of Veterinary Medicine [Internet]. 2008 [Cited 24 Nov 2019];6(1):121-26. https://doi.org/10.3329/bjvm.v6i1.1350v
Scorzoni L, Benaducci T, Ameida AMF, Silva DHS, Da Silva BV, Gianinni MJSM. The use of standard methodology for the determination of antifungal activity of natural products against medical yeast Candida sp and Cryptococcus sp. Brazillian Journal of Microbiology [Internet]. 2007 [cited 30 June 2018];38(1):391-97. https://doi.org/10.1590/S1517-83822007000300001
Magaldi S, Mata- essayag S, Hartung de capriles C, Perez C, Colella MT, Olaizola C et al. Well diffusion for antifungal susceptibility testing. Int J Infect Dis [Internet]. 2004 [cited 30 June 2018];1(8):39-45. https://doi.org/10.1016/j.ijid.2003.03.002
Lakshmanan K, Arumungam A, Mani R. Phytochemical screening and in – vitro antimicrobial activity of Vitex negundo L. var. purpurascens Sivar and Mold against pathogenic microorganisms. Drug Invention Today. 2012;4(12):667.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. Journal of Pharmaceutical Analysis. 2016; (6): 71 – 79. https://doi.org/10.1016/j.jpha.2015.11.005
Alastruey-Izquierdo A, Melhem M, Bonfietti L, Rodriguez-Tudela J. Susceptibility Test for Fungi: Clinical and Laboratorial Correlations in Medical Mycology. Rev Inst Med Trop Sao Paulo. 2015;57(19): 57–64. https://doi.org/10.1590/s0036-46652015000700011
Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. International Journal of Food Science and Technology. 2002; (37): 153-61. https://doi.org/10.1046/j.1365-2621.2002.00552.x
Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry. 2002;50(10):3010-14. https://doi.org/10.1021/jf0115589
Gajula D, Verghese M, Boateng J, Walker LT, Shackelford L, Mentreddy SR, Cedric S. Determination of Total Phenolics, Flavonoids and Antioxidant and Chemopreventive Potential of Basil (Ocimum basilicum L. and Ocimum tenuiflorum L.). International Journal of Cancer Research. 2009;5:130-43. https://dx.doi.org/10.3923/ijcr.2009.130.143
Andrews JM. Determination of Minimum Inhibitory Concentrations. Journal of Antimicrobial Chemotherapy. 2001;49(6):5-16. https://doi.org/10.1093/jac/48.suppl_1.5
Sen P, Sahu PK, Haldar R, Kumar Sahu K, Prasad P, Roy A. Apigenin Naturally Occurring Flavonoids: Occurrence and Bioactivity. Pharmaceutical and Biosciences Journal. 2016; 4(6): 56-68. http://dx.doi.org/10.20510/ukjpb/4/i6/134666
Wang Q, Xie M. Antibacterial activity and mechanism of luteolin on Staphylococcus aureus. National Center for Biotechnology Information. 2010; 50(9): 1180-84.
Lou Z, Wang H, Ra S, Sun J, Ma C, Li J. P-Coumaric Acid Kills Bacteria through Dual Damage Mechanisms. Food Control. 2012; 25 (2): 550-54. https://doi.org/10.1016/j.foodcont.2011.11.022
Valentino MJG, Ganado LS, Ganado MR, Undan JR. Phytochemical screening and bio assay of the anti-microbial activity of three species of bamboo in Nueva Ecija, Philippines. Advances in Environmental Biology. 2015;9(24):389-96.
Austria KC, Waing KGW, Valentino MJ. Anti-oxidant and antibacterial potentials of Bambusa blumeana JA and JHP Schultes and Bambusa vulgaris Schrad. ex Wendl. International Journal of Biology, Pharmacy and Allied Sciences. 2017;6(11):2175-88.
Zhang JJ, Liu M, Li Y, Zhou T, Xu DP. Nutritional values and biological activities of bamboo shoots and leaves. International Journal of Food and Nutrition Safety. 2016;7(2):98-108.
Wangawar SN, Shendarkar GR, Shelke DP, Daswad AK, Pohare JG, Roge AB. Phytochemical screening and antimicrobial activity of Dendrocalamus strictus leaves extracts. World Journal of Pharmaceutical Research. 2017; 6(4):1029-41. https://doi.org/10.20959/wjpr20174-8128
Owolabi MS, Lajide Preliminary Phytochemical screening and antimicrobial activity of crude extracts of Bambusa vulgaris Schrad. ex J.C. Wendl. (Poaceae) from Southwestern Nigeria. American Journal of Essential Oils and Natural Products. 2015;3(1):42-45.
Cushnie T, Lamb JA. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents [Internet]. 2005 [cited 30 June 2018];26(5):343-56. http://dx.doi.org/10.1016/j.ijantimicag.2005.09.002
Wafa N, Sofiane G, Mouhamed K. The antioxidant and antimicrobial activities of flavonoids and tannins extracted from Phlomis bovei De Noé. European Journal of Experimental Biology. 2016;6(3):55-61.
Saxena M, Nema R, Jyoti S, Dharmendra S, Abishek G. Phytochemistry of Medicinal Plants. Journal of Pharmacognosy and Phytochemistry. 2013;1(6):168-82.
Huang Y, Xiao D, Burton-Freeman BM, Edirisinghe I. Chemical changes of bioactive phytochemicals during thermal processing. Reference Module in Food Science. 2015. https://doi.org/10.1016/B978-0-08-100596-5.03055-9
Lila MA, Raskin I. Health related interactions of phytochemicals. Journal of Food Science. 2006;70(1):20-27. https://doi.org/10.1111/j.1365-2621.2005.tb09054.x
Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Current Drug Targets. 2011;12(1):122-32. https://doi.org/10.2174/138945011793591626
Sultana B, Anwar F, Ashraf M. Effect of extraction solvent or technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14(6):2167-80. https://doi.org/10.3390/molecules14062167
Downloads
Published
Versions
- 01-07-2022 (2)
- 17-04-2022 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Abegail G Saducos

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









