Indigenous entomopathogenic nematode as biocontrol agents for insect pest management in hilly regions
DOI:
https://doi.org/10.14719/pst.1501Keywords:
Agrotis segetum, Biocontrol, Helicoverpa armigera, Mythimna separata, Spodoptera lituraAbstract
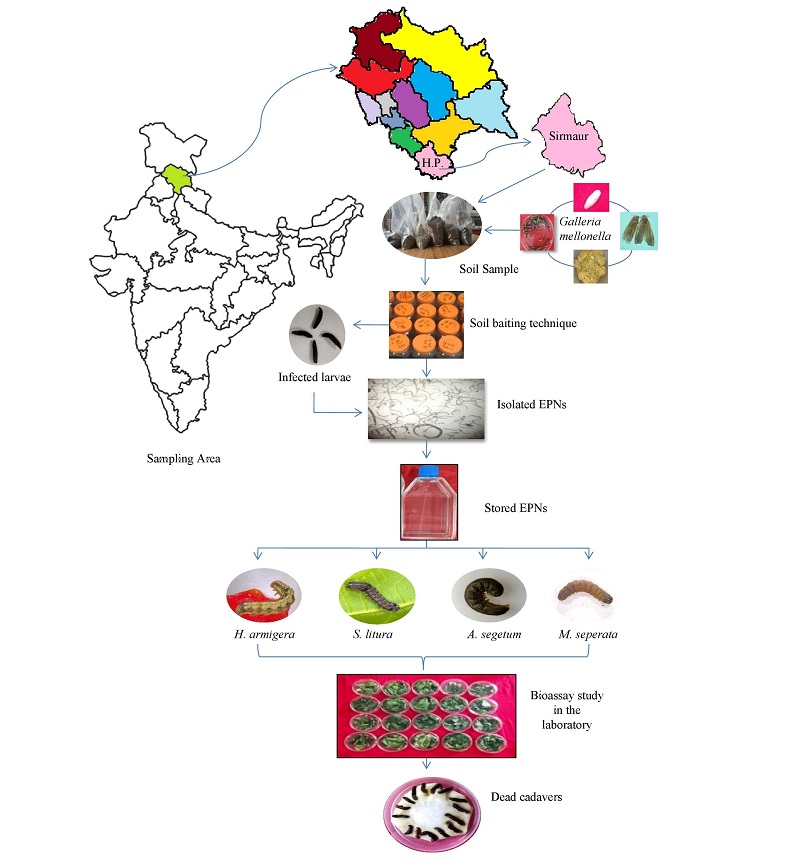
The present investigation mainly emphasized on the development and use of entomopathogenic nematodes (EPNs) as a bio-insecticide. The success in controlling insect pests in the soil environments increased the production and use of the adapted indigenous EPNs species for insect management in the fields. EPNs as biocontrol agents were capable for high virulence, easy for application, safe for non-target animals and eco-friendly in nature. These nematodes have ubiquitous nature. These occur in low population in their natural habitat which was mass multiplied in the laboratory. In the present investigation, 5 concentrations (30IJs, 60IJs, 90IJs, 120IJs and 150IJs) of Heterorhabditis bacteriophora strain S15 were applied against the 3rd and 4th instar larvae of four major agricultural insect pests, namely Helicoverpa armigera, Spodoptera litura, Agrotis segetum and Mythimna separata under laboratory conditions at different time exposure (24, 48, 72 and 96 hr). It was observed that the 3rd and 4th larval instars of all 4 insects (H. armigera, S. litura, A. segetum and M. separata) were highly susceptible for the pathogenesis caused by H. bacteriophora Sirmaur isolates. Amongst all insects, both the larval instars of M. separata are highly susceptible for EPNs infection with highest 96% and 98% mortality in highest dose @150IJs. In 3rd instar larvae of other insects such as H. armigera, S. litura and A. segetum larval mortality ranges from 84%, 92% and 94% respectively. Among 4th instar larvae of H. armigera, S. litura and A. segetum the pathogenicity varies from 88%, 94% and 96% respectively. The recorded median lethal concentration (LC50) in 3rd instar larvae of H. armigera, S. litura, A. segetum and M. separata varies from 36.15, 30.05, 30.97 and 23.8. Similarly in 4th instar larvae of H. armigera, S. litura, A. segetum and M. separata, LC50 ranged from 31.41, 28.64, 26.92 and 20.64 respectively. Statistically significant variations were observed in the data recorded on the mortality, in all the treatments. EPNs are the best weapon to overcome insect resistance problems and must be employed to manage insect population.
Downloads
References
Kaneda T, Greenbaum C, Kline K. World population data sheet shows older populations growing, total fertility rates declining. Population Reference Bureau. 2020. Available from: https://www.prnewswire.com/news-releases/prbs-2021-world-population-data-sheet-released-301357333.html
Thakur N, Tomar P, Kaur S, Jhamta S, Thakur R, Yadav AN. Entomopathogenic soil microbes for sustainable crop protection, In: Yadav AN.; Editor. Soil Microbiomes for Sustainable Agriculture Springer. 2021;529-71. https://doi.org/10.1007/978-981-15-6949-4_1
Bhat AH, Chaubey AK, Askary TH. Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt J Biol Pest Co. 2020;30:1-15. https://doi.org/10.1186/s41938-020-0212-y
Kaya HK, Gaugler R. Entomopathogenic nematodes. Ann Rev Ent. 1993; 38(1):181-206. https://doi.org/10.1146/annurev.en.38.010193.001145
Hominick WM. Biogeography. In: Gaugler R., editor Entomopathogenic Nematology. CABI Publications, UK. 2002;1:115-43.https://doi.org/10.1079/9780851995670.0115
Campos-Herrera R. Nematode pathogenesis of insects and other pests: ecology and applied technologies for sustainable plant and crop protection In: Campos-Herrera R., editor Sustainability in plant and crop protection. Springer. 2015; p. 1-531. https://doi.org/10.1007/978-3-319-18266-7
Georgis R, Koppenhöfer AM, Lacey LA, Bélair G, Duncan LW, Grewal PS et al. Successes and failures in the use of parasitic nematodes for pest control. Biol Control. 2006;38(1):103-23. https://doi.org/10.1016/j.biocontrol.2005.11.005
Grewal PS, Selvan S, Gaugler R. Nematodes: niche breadth for infection, establishment and reproduction. J Therm Biol. 1994;19:245-53.https://doi.org/10.1016/0306-4565(94)90047-7
Gulcu BA, Cimen HA, Raja RK, Hazir SE. Entomopathogenic nematodes and their mutualistic bacteria: their ecology and application as microbial control agents. Biopestic. 2017;13(2):79-112.
Kaya HK, Nelsen C. Encapsulation of steinernematid and heterorhabditid nematodes with calcium alginate: a new approach for insect control and other applications. Environ Entomol. 1985;14(5):572-74. https://doi.org/10.1093/ee/14.5.572
Thakur N, Kaur S, Tomar P, Thakur S, Yadav AN. Microbial biopesticides: current status and advancement for sustainable agriculture and environment, In: Rastegari AA, Yadav AN, Yadav N, editors Trends of Microbial Biotechnology for Sustainable Agriculture and Biomedicine Systems: Diversity and Functional Perspectives. Elsevier, Amsterdam. p 243-82. https://doi.org/10.1016/B978-0-12-820526-6.00016-6
Dillman AR, Guillermin ML, Lee JH, Kim B, Sternberg PW, Hallem EA. Olfaction shapes host–parasite interactions in parasitic nematodes. In: Proceedings of the National Academy of Sciences. 2012;109(35):E2324-E2333. https://doi.org/10.1073/pnas.1211436109
Hallem EA, Dillman AR, Hong AV, Zhang Y, Yano JM, DeMarco SF, Sternberg PW. A sensory code for host seeking in parasitic nematodes. Current Biol. 2011;21(5):377-83. https://doi.org/10.1016/j.cub.2011.01.048
Grewal P, Gaugler R, Selvan S. Host recognition by entomopathogenic nematodes: behavioral response to contact with host feces. J Chem Ecol. 1993;19(6):1219-31. https://doi.org/10.1007/BF00987382
Burnell A, Stock SP. Heterorhabditis, Steinernema and their bacterial symbionts—lethal pathogens of insects. Nematol. 2000;2(1):31-42. https://doi.org/10.1163/156854100508872
Forst S, Dowds B, Boemare N, Stackebrandt E. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu Rev Microbiol. 1997;51(1):47-72. https://www.annualreviews.org/doi/10.1146/annurev.micro.51.1.47
Fitt GP. The ecology of Heliothis species in relation to agroecosystems. Annu Rev Entomol. 1989;34(1):17-53.https://doi.org/10.1146/annurev.en.34.010189.000313
Dhir B, Mohapatra H, Senapati B. Assessment of crop loss in groundnut due to tobacco caterpillar, Spodoptera litura (F.). Indian J Plant Prot. 1992;20(2):215-17.
Rao GR, Wightman J, Rao DR. World review of the natural enemies and diseases of Spodoptera litura (F.) (Lepidoptera: Noctuidae). Int J Tropic Insect Sci. 1993;14(3):273-84. https://doi.org/10.1017/S1742758400014764
Ram G, Misra S, Dhamayanthi K. Relative susceptibility of advanced hybrids and promising cultivars of potato, Solanum tuberosum L. to greasy cutworm, Agrotis ipsilon (Hufn.) in North-eastern plains. J Entomol Res. 2001;25(3):183-87.
Mrowczynski M, Wachowiak H, M B. Cutworms -a dangerous pest in the autumn of 2003. Ochrana Roslin. 2003;47:24-26.
Napiorkowska K, Gawowska J. Increase of harmfulness of caterpillars (Hadeninae and Noctuinae, Lepidoptera: Noctuidae) on cabbage and other cole crops. Progress in Plant Prot. 2004;44:978-80.
Arab Republic of Egypt. Ministry of Agriculture and Land Reclamation. Technical Recommendations for Agricultural Pest Control. p. 1-285. http://www.apc.gov.eg/Files/Releases/Recom.20-English.pdf
Hill M, Atkins A. Incidence of the armyworm, Mythimna separata Walker (Noctuidae: Lepidoptera), and its introduced parasite, Apanteles ruficrus Halliday (Braconidae: Hymenoptera), in maize. New Zeal J Agr Res. 1983;26(1):135-38. https://doi.org/10.1080/00288233.1983.10420963
White G. A method for obtaining infective nematode larvae from cultures Science (Washington). 1927;66(1709):302-03. https://doi.org/10.1126/science.66.1709.302-b
Orozco RA, Lee M-M, Stock SP. Soil sampling and isolation of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae). J Visual Exp. 2014;(89):e52083. https://dx.doi.org/10.3791/52083
Kumar L, Bisht RS, Singh H, Kumar M. Studies on growth and development of Helicoverpa armigera (Hub.) on various hosts and artificial diet under laboratory conditions. Int J Curr Microbiol Appl Sci. 2017;6(12):1627-37. https://doi.org/10.20546/ijcmas.2017.612.183
Seth R, Sharma V. Growth, development, reproductive competence and adult behaviour of Spodoptera litura (Lepidoptera: Noctuidae) reared on different diets. 2002;15-22. https://www.osti.gov/etdeweb/biblio/20246472
Verma K. Bioecology and management of turnip moth (Lepidoptera, Noctidae) attacking vegetable crops. Doctoral dissertation, Ph. D. Thesis, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Solan, 1996 p. 121.
Manjula K, Kotikal Y. Biology of turnip moth, Agrotis segetum (Denis and Schiffermüller) on palak, Beta vulgaris var. bengalensis Hort. J Entomol Zool Stud. 2018;6(6):1183-86.
Patil J, Vijayakumar R, Linga V, Sivakumar G. Susceptibility of Oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae) larvae and pupae to native entomopathogenic nematodes. J Appl Entomol. 2020;144(7):647-54. https://doi.org/10.1111/jen.12786
Hassani-Kakhki M, Karimi J and Hosseini M. Efficacy of entomopathogenic nematodes against potato tuber moth, Phthorimaea operculella (Lepidoptera: Gelechiidae) under laboratory conditions. Biocontrol Sci Technol. 2013;23(2):146-59. https://doi.org/10.1080/09583157.2012.745481
Vashisth S. Distribution and biocontrol potential of entomopathogenic nematodes against some lepidopterous pests. Doctoral dissertation, Ph. D. Thesis, CSKPV Krishi Vishavavidyalaya, Palampur. Himachal Pradesh. 2014;1-182. http://hdl.handle.net/10603/203884
Kary NE, Golizadeh A, Dastjerdi HR, Mohammadi D, Afghahi S, Omrani M, et al. A laboratory study of susceptibility of Helicoverpa armigera (Hübner) to three species of entomopathogenic nematodes. Munis Entomol Zool. 2012;7(1):72-379.
Vashisth S, Chandel Y, R Chandel. Comparative efficacy of indigenous heterorhabditid nematodes from north western Himalaya and Heterorhabditis indica (Poinar, Karunakar & David) against the larvae of Helicoverpa armigera (Hubner). Int J Pest Manage. 2019;65(1):16-22. https://doi.org/10.1080/09670874.2018.1453099
Andaló V, Faria LS, Carvalho FJ, Assis GA, Santos V, Mendes SM, Gonring AH Entomopathogenic nematodes for the control of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) pupae. Arq Inst Biol. 2021;88:1-8. https://doi.org/10.1590/1808-1657000742019
Glazer I, Navon A. Activity and persistence of entomoparasitic nematodes tested against Heliothis armigera (Lepidoptera: Noctuidae). J Econ Entomol. 1990;83(5):1795-1800. https://doi.org/10.1093/jee/83.5.1795
Yan X, Shahid Arain M, Lin Y, Gu X, Zhang L, Li J, Han R. Efficacy of entomopathogenic nematodes against the tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). J Econ Entomol. 2020;113(1):64-72. https://doi.org/10.1093/jee/toz262
Acharya R, Hwang HS, Mostafiz MM, Yu YS, Lee KY. Susceptibility of various developmental stages of the fall armyworm, Spodoptera frugiperda, to entomopathogenic nematodes. Insects. 2020;11(12):868. https://doi.org/10.3390/insects11120868
Acharya R, Yu YS, Shim JK, Lee KY. Virulence of four entomopathogenic nematodes against the tobacco cutworm, Spodoptera litura Fabricius. Biol Control. 2020;150:104348. https://doi.org/10.1016/j.biocontrol.2020.104348
Dichusa CA, Ramos R, Aryal S, Sumaya NP, Sumaya NH. Survey and identification of entomopathogenic nematodes in the province of Cotabato, Philippines, for biocontrol potential against the tobacco cutworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Egypt J Biol Pest Co. 2021;31(1):1-10. https://doi.org/10.1186/s41938-021-00390-w
Radhakrishnan S, Shanmugam S. Bioefficacy of entomopathogenic nematodes against Spodoptera litura (Lepidoptera: Noctuidae) in Bhendi. Int J Curr Microbiol Appl Sci. 2017;6:2314-19. DOI:10.20546/ijcmas.2017.607.273.https://doi.org/10.20546/ijcmas.2017.607.273
Chandel Y, Kapoor S, Kumar S. Virulence of Heterorhabditis bacteriophora (Poinar) against cutworm, Agrotis segetum (Denis & Schiff.). J Biol Cont. 2010;23(4):409-15. https://doi.org/10.18311/jbc/2009/3696
Kumari V, Singh NP, Shinde S, Meena S, Lata RK. Factors affecting propagation of Heterorhabditis bacteriophora Poinar, an entomopathogenic nematode in Plutella xylostella (L.) J Biopestic. 2019;12(1):1-6.
Chandel RS, Verma KS, Rana A, Sanjta S, Badiyala A, Vashisth S, et al. The ecology and management of cutworms in India. Oriental Insects. 2021;1-26. https://doi.org/10.1080/00305316.2021.1936256
Radhakrishnan S, Shanmugam S, Ramasamy R. Bio control efficacy of Entomopathogenic nematodes against Black Cutworms, Agrotis ipsilon (Hufnagel) (Noctuidae: Lepidoptera) in potato. Chem Sci Rev Lett. 2017;6(21):219-24. https://www.researchgate.net/publication/318458813
Ebrahimi L, TanhaMaafi Z, Sharifi P. First report of the entomopathogenic nematode, Steinernema carpocapsae from Moghan region of Iran and its efficacy against the turnip moth, Agrotis segetum Denis & Schiffermuller (Lepidoptera: Noctuidae), larvae. Egypt J Biol Pest Co. 2019;29(1):1-6. https://doi.org/10.1186/s41938-019-0168-y
FAO. 2018 http//www.fao.org/statisticaldatabases/agriculture.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Preety Tomar, Neelam Thakur, Ajar Nath Yadav

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









