Modulation by S-nitrosoglutathione (a natural nitric oxide donor) of photosystem in Pisum sativum leaves, as revealed by chlorophyll fluorescence: Light-dependent aggravation of nitric oxide effects
DOI:
https://doi.org/10.14719/pst.2248Keywords:
Chlorophyll fluorescence, Nitric oxide, Photosynthesis, Photosystems, Respiration, High lightAbstract
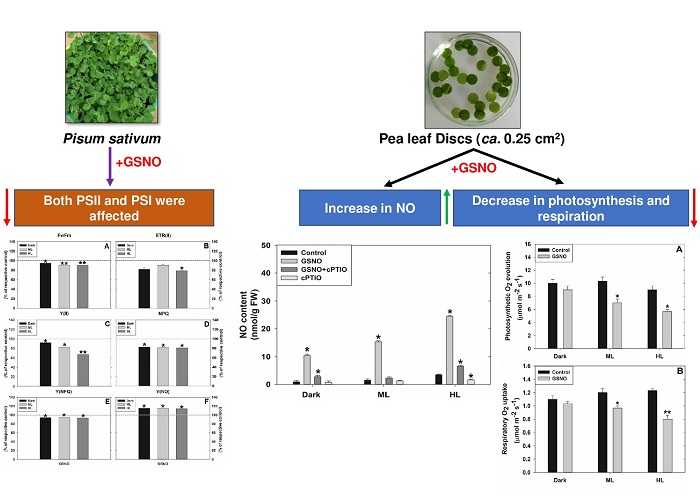
The reported effects of nitric oxide (NO), a signaling molecule, on the photochemical components of leaves are ambiguous. We examined the changes by a natural NO donor, S-nitrosoglutathione (GSNO). The effect of GSNO on Pisum sativum leaves was studied after a 3-hour exposure in dark, moderate (ML), or high light (HL). The NO levels in GSNO-treated samples were at their maximum under HL, compared to those under ML or dark. Most of the elevated NO was decreased by 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), a NO scavenger, confirming the NO increase. Treatment with GSNO caused inhibition of photosynthesis/respiration and restricted electron transport mediated by both photosystem (PS)II and PSI. However, the inhibition by NO-donor of PSII components was stronger than those of PSI. A marked increase in the PSI acceptor side limitation [Y(NA)] and a decrease in PSI donor side limitation [Y(ND)] indicated an upregulation of cyclic electron transport, possibly to balance the damage to PSII by GSNO. We suggest that NO aggravated the HL-induced inhibition of photosynthesis and dark respiration.
Downloads
References
Gururani MA, Venkatesh J, Tran LS. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Molecular Plant. 2015; 8(9):1304-1320. https://doi.org/10.1016/j.molp.2015.05.005
Szyma?ska R, ?lesak I, Orzechowska A, Kruk J. Physiological and biochemical responses to high light and temperature stress in plants. Environmental and Experimental Botany. 2017; 139:165-177. https://doi.org/10.1016/j.envexpbot.2017.05.002
Singhal RK, Jatav HS, Aftab T, Pandey S, Mishra UN, Chauhan J, Chand S, Saha D, Dadarwal BK, Chandra K, Khan MA. Roles of nitric oxide in conferring multiple abiotic stress tolerance in plants and crosstalk with other plant growth regulators. Journal of Plant Growth Regulation. 2021; 40(6):2303-2328. https://doi.org/10.1007/s00344-021-10446-8
Wani KI, Naeem M, Castroverde CDM, Kalaji HM, Albaqami M, Aftab T. Molecular mechanisms of nitric oxide (NO) signaling and reactive oxygen species (ROS) homeostasis during abiotic stresses in plants. International Journal of Molecular Sciences. 2021; 22(17):9656. https://doi.org/10.3390/ijms22179656
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends in Plant Science. 2011; 16(6):300-309. https://doi.org/10.1016/j.tplants.2011.03.007
Sunil B, Rajsheel P, Aswani V, Bapatla RB, Talla SK, Raghavendra AS. Photosynthesis is sensitive to nitric oxide and respiration sensitive to hydrogen peroxide: Studies with pea mesophyll protoplasts. Journal of Plant Physiology. 2020a; 246-247:153133. https://doi.org/10.1016/j.jplph.2020.153133
Altaf MA, Shahid R, Ren MX, Naz S, Altaf MM, Khan LU, Tiwary, RK, Lal MK, Shahid MA, Kumar R, Nawaz, MA, Jahan MS, Jan BL, Ahmad P. Melatonin improves drought stress tolerance of tomato by modulation plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants. 2022; 11(2):309. https://doi.org/10.3390/antiox11020309
Wu ZX, Xu NW, Yang M, Li XL, Han JL, Lin XH, Yang Q, Lv GH, Wang J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environmental Science and Pollution Research International. 2022; 29(25):37248-37265. https://doi.org/10.1007/s11356-022-18641-0
Lopes-Oliveira PJ, Oliveira HC, Kolbert Z, Freschi L. The light and dark sides of nitric oxide: multifaceted roles of nitric oxide in plant responses to light. Journal of Experimental Botany. 2021; 72(3):885-903. https://doi.org/10.1093/jxb/eraa504
Zhou X, Joshi S, Khare T, Patil S, Shang J, Kumar V. Nitric oxide, crosstalk with stress regulators and plant abiotic stress tolerance. Plant Cell Reports. 2021; 40(8):1395-1414. https://doi.org/10.1007/s00299-021-02705-5
Arasimowicz M, Floryszak-Wieczorek J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Science. 2007;172:876887. https://doi.org/10.1016/j.plantsci.2007.02.005
Bhardwaj S, Kapoor D, Singh S, Gautam V, Dhanjal DS, Jan S, Ramamurthy PC, Prasad R, Singh J. Nitric Oxide: A ubiquitous signal molecule for enhancing plant tolerance to salinity stress and their molecular mechanisms. Journal of Plant Growth Regulation. 2021; 40(6):2329-2341. https://doi.org/10.1007/s00344-021-10394-3
Wodala B, Deák Z, Vass I, Erdei L, Horváth F. Nitric oxide modifies photosynthetic electron transport in pea leaves. Acta BiologicaSzegediensis. 2005; 49(1-2):7-8. https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2400
Wodala B, Horváth F. The effect of exogenous NO on PSI photochemistry in intact pea leaves. Acta BiologicaSzegediensis. 2008; 52(1):243-245. https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2634
Sahay S, De La Cruz Torres E, Robledo-Arratia L, Gupta M. Photosynthetic activity and RAPD profile of polyethylene glycol treated B. juncea L. under nitric oxide and abscisic acid application. Journal of Biotechnology. 2020; 313:29-38. https://doi.org/10.1016/j.jbiotec.2020.03.004
Hajihashemi S, Skalicky M, Brestic M, Pavla V. Effect of sodium nitroprusside on physiological and anatomical features of salt-stressed Raphanus sativus. Plant Physiology and Biochemistry. 2021; 169:160-170. https://doi.org/10.1016/j.plaphy.2021.11.013
Wodala B, Deák Z, Vass I, Erdei L, Altorjay I, Horváth F. In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiology. 2008; 146(4):1920-1927. https://doi.org/10.1104/pp.107.110205
Yang JD, Zhao HL, Zhang TH, Yun JF. Effects of exogenous nitric oxide on photochemical activity of photosystem II in potato leaf tissue under non-stress condition. Acta Botanica Sinica. 2004; 46(9):1009-1014. https://www.jipb.net/EN/Y2004/V46/I9/1009
Sunil B, Strasser RJ, Raghavendra AS. Targets of nitric oxide (NO) during modulation of photosystems in pea mesophyll protoplasts: studies using chlorophyll a fluorescence. Photosynthetica. 2020b; 58(SI):452-459.https://doi.org/10.32615/ps.2019.183
Aswani V, Rajsheel P, Bapatla RB, Sunil B, Raghavendra AS. Oxidative stress induced in chloroplasts or mitochondria promotes proline accumulation in leaves of pea (Pisum sativum): another example of chloroplast-mitochondria interactions. Protoplasma. 2019; 256(2):449-457. https://doi.org/10.1007/s00709-018-1306-1
Zhou B, Guo Z, Xing J, Huang B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthesguianensis. Journal of Experimental Botany. 2005; 56(422):3223-3228. https://doi.org/10.1093/jxb/eri319
Talla S, Riazunnisa K, Padmavathi L, Sunil B, Rajsheel P, Raghavendra AS. Ascorbic acid is a key participant during the interactions between chloroplasts and mitochondria to optimize photosynthesis and protect against photoinhibition. Journal of Biosciences. 2011; 36(1):163-173. https://doi.org/10.1007/s12038-011-9000-x
Schreiber U, Klughammer C. New accessory for the DUAL-PAM-100: The P515/535 module and examples of its application. PAM Application Notes. 2008; 1:1-10. (2nd ed). http://www.walz.com/
Pandey J, Devadasu E, Saini D, Dhokne K, Marriboina S, Raghavendra AS, Subramanyam R. Reversible changes in structure and function of photosynthetic apparatus of pea (Pisum sativum) leaves under drought stress. The Plant Journal. 2023; 113:60-74.https://doi.org/10.1111/tpj.16034
Ederli L, Reale L, Madeo L, Ferranti F, Gehring C, Fornaciari M, Romano B, Pasqualini S. NO release by nitric oxide donors in vitro and in planta. Plant Physiology and Biochemistry. 2009; 47(1):42-48. https://doi.org/10.1016/j.plaphy.2008.09.008
Antoniou C, Filippou P, Mylona P, Fasoula D, Ioannides I, Polidoro A, Fotopoulos V. Developmental stage- and concentration-specific sodium nitroprusside application results in nitrate reductase regulation and the modification of nitrate metabolism in leaves of Medicagotruncatula plants. Plant Signaling and Behavior. 2013; 8(9):e25479. https://doi.org/10.4161/psb.25479
Esmail NY, Hashem HA, Hassanein AA. Effect of treatment with different concentrations of sodium nitroprusside on survival, germination, growth, photosynthetic pigments and endogenous nitric oxide content of Lupines termis L. plants. Acta Scientific Agriculture (ISSN: 2581-365X). 2018; 2(5):48-52.
Zottini M, Formentin E, Scattolin M, Carimi F, Lo Schiavo F, Terzi M. Nitric oxide affects plant mitochondrial functionality in vivo. FEBS Letters. 2002; 515(1-3):75-78.https://doi.org/10.1016/s0014-5793(02)02438-9
Pandey S, Kumari A, Shree M, Kumar V, Singh P, Bharadwaj C, Loake GJ, Parida SK, Masakapalli SK, Gupta KJ. Nitric oxide accelerates germination via the regulation of respiration in chickpea. Journal of Experimental Botany. 2019; 70(17):4539-4555. https://doi.org/10.1093/jxb/erz185
Ganjewala D, Bobba S, Raghavendra AS. Sodium nitroprusside affects the level of anthocyanin and flavonol glycosides in pea (Pisum sativum L. cv. Arkel) leaves. Acta Biologica Szegediensis. 2008; 52(2):301-305. https://abs.bibl.u-szeged.hu/index.php/abs/article/view/
Poderoso JJ, Helfenberger K, Poderoso C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide. 2019; 88:61-72. https://doi.org/10.1016/j.niox.2019.04.005
Misra AN, Vladkova R, Singh R, Misra M, Dobrikova AG, Apostolova EL. Action and target sites of nitric oxide in chloroplasts. Nitric oxide. 2014;39:35-45. https://doi.org/10.1016/j.niox.2014.04.003
Chang HL, Hsu YT, Kang CY, Lee TM. Nitric oxide down-regulation of carotenoid synthesis and PSII activity in relation to very high light-induced singlet oxygen production and oxidative stress in Chlamydomonas reinhardtii. Plant and Cell Physiology. 2013; 54(8):1296-1315. https://doi.org/10.1093/pcp/pct078
Vladkova R, Dobrikova AG, Singh R, Misra AN, Apostolova E. Photoelectron transport ability of chloroplast thylakoid membranes treated with NO donor SNP: changes in flash oxygen evolution and chlorophyll fluorescence. Nitric Oxide. 2011; 24(2): 84-90. https://doi.org/10.1016/j.niox.2010.12.003
Ördög A, Wodala B, Rózsavölgyi T, Tari I, Horváth F. Regulation of guard cell photosynthetic electron transport by nitric oxide. Journal of Experimental Botany. 2013; 64(5):1357-1366. https://doi.org/10.1093/jxb/ers397
Murata N, Allakhverdiev SI, Nishiyama Y. The mechanism of photoinhibition in vivo: re-evaluation of the roles of catalase, ?-tocopherol, non-photochemical quenching, and electron transport. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2012; 1817(8):1127-1133. https://doi.org/10.1016/j.bbabio.2012.02.020
Tikkanen M, Mekala NR, Aro EM. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochimica et Biophysica Acta (BBA)- Bioenergetics. 2014; 1837(1):210-215. https://doi.org/10.1016/j.bbabio.2013.10.001
Six C, Sherrard R, Lionard M, Roy S, Campbell DA. Photosystem II and pigment dynamics among ecotypes of the green alga Ostreococcus. Plant Physiology. 2009; 151(1):379-390. https://doi.org/10.1104/pp.109.140566
Xu Y, Sun X, Jin J, Zhou H. Protective effect of nitric oxide on light-induced oxidative damage in leaves of tall fescue. Journal of Plant Physiology. 2010; 167(7):512-518. https://doi.org/10.1016/j.jplph.2009.10.010
Solymosi D, Shevela D, Allahverdiyeva Y. Nitric oxide represses photosystem II and NDH-1 in the cyanobacterium Synechocystis sp. PCC 6803. Biochimica et Biophysica Acta (BBA)- Bioenergetics.2022;1863(1):148507.https://doi.org/10.1016/j.bbabio.2021.148507
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002;110(3):361-371. https://doi.org/10.1016/s0092-8674(02)00867-x
Downloads
Published
Versions
- 01-04-2023 (2)
- 15-01-2023 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Deepak Saini, Ramesh B Baptala, Jayendra Pandey, Vetcha Aswani, Bobba Sunil, Shashibhushan Gahir, Pulimamidi Bharath, Rajagopal Subramanyam, Agepati S Raghavendra

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









