Identification of disease suppressive potential of Trichoderma virens and Jasmonic acid against fusarium wilt and damping-off in “Seed Primed” tomato plants
DOI:
https://doi.org/10.14719/pst.2325Keywords:
Biotic stress, Seed priming, Biocontrol, Disease incidence, Plant defenceAbstract
Disease causing phytopathogens are responsible for an approximately 15% reduction in worldwide food production. Therefore, for efficient management of plant diseases, a systematic understanding of the harmful impacts of pathogens on economic crops is essential. The practice of sustainable agriculture aims at the development of a system that supports the growth of plants but simultaneously induces adverse effects on the existence of pathogens. Therefore, the current research was designed to monitor the seed priming effects of Trichoderma virens (as Biocontrol Agent, BCA) and Jasmonic acid (a chemical inducer) in tomato plants infected with two devastating soil-borne pathogens viz., Fusarium oxysporum lycopersici (Fol) and Rhizoctonia solani. Application of these agents in infected plants alone or together leads to the establishment of various disease-suppressive mechanisms in the host plants as observed in the form of enhanced seedling vigour index, percentage germination, morphological growth, and a substantial decrease in the percentage of disease incidence. Furthermore, pathogen inoculation in diseased plants enhances the content of two compatible osmolytes i.e., proline and glycine betaine which themselves serve as defensive molecules by acting as osmoprotectants and signalling molecules in the induction of various defence-related pathways in the stressed plants. Our study provides important insights into the effectiveness of T. virens and JA in the amelioration of pathogen-induced damage in the host plants. The
inferences obtained from this research highlight the better efficiency of combined applications of T. virens and JA against these two soil-borne pathogens.
Downloads
References
Panno S, Davino S, Caruso AG, Bertacca S, Crnogorac A, Mandi? A et al. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the Mediterranean basin. Agronomy 2021 Nov 1 ;11(11):2188. https://doi.org/10.3390/agronomy11112188
Joshi R. A review of Fusarium oxysporum on its plant interaction and industrial use. J Med Pl Studies. 2018;6(3):112-15. https://doi.org/10.22271/plants.2018.v6.i3b.07
Clocchiatti A, Hannula SE, Rizaludin MS, Hundscheid MPJ, Klein Gunnewiek PJA, Schilder MT et al. Impact of cellulose-rich organic soil amendments on growth dynamics and pathogenicity of Rhizoctonia solani. Microorganisms. 2021 Jun 1;9(6). https://doi.org/10.3390/microorganisms9061285
Ghosh UK, Islam MN, Siddiqui MN, Khan MAR. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal Behav Aug;16(8):1913306. https://doi.org/10.1080/15592324.2021.1913306
Giri J. Glycinebetaine and abiotic stress tolerance in plants.Plant Signal Behav 2011 Nov 6(11):1746-51
Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA et al. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol 1;122(3):747-56
Suprasanna P, Rai AN, Hima Kumari P, Kumar SA, Kavi Kishor PB. Modulation of proline: implications in plant stress tolerance and development. In: Anjum NA, Gill SS, Gill R, editors. Plant Adaptation to Environmental UK: CABI Publishers. 2014; p. 68-93.
Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, Brtnicky M et al. Impact of agrochemicals on soil microbiota and management: A review. 2020 Jan 23;9(2):34. https://doi.org/10.3390/land9020034
Panth M, Hassler SC, Baysal-Gurel F. Methods for management of soilborne diseases in crop production. Agriculture. 2020 Jan 11;10(1):16. https://doi.org/10.3390/agriculture10010016
Collinge DB, Jensen DF, Rabiey M, Sarrocco S, Shaw MW, Shaw RH. Biological control of plant diseases – What has been achieved and what is the direction?. Plant Pathol 2022 Jun 1. https://doi.org/10.1111/ppa.13555
Fenibo EO, Ijoma GN, Matambo T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front Sustain Food Syst. 2021 Jun 11;5:141. https://doi.org/10.3389/fsufs.2021.619058
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, el Hamss H et al. Biological control of plant pathogens: A global perspective. Microorganisms.2022. https://doi.org/10.3390/microorganisms10030596
Sood M, Kapoor D, Kumar V, Sheteiwy MS, Ramakrishnan M, Landi M et al. Trichoderma: The “Secrets” of a multitalented biocontrol agent. Plants (Basel). 2020
Mishra J, Dutta V, Arora NK. Biopesticides in India: technology and sustainability linkages. 3 Biotech. 2020 May 1. https://doi.org/10.1007/s13205-020-02192-7
Zhang L, Zhang F, Melotto M, Yao J, He SY. Jasmonatesignaling and manipulation by pathogens and insects. J Exp Bot. 2017 Mar 3. https://doi.org/10.1093/jxb/erw478
Wang Y, Mostafa S, Zeng W, Jin B. Function and mechanism of jasmonicacid in plant responses to abiotic and biotic stresses. Int J Mol Sci. 2021 Aug. https://doi.orger/10.3390/ijms22168568
Morton DJ, Stroube WH. Antagonistic and stimulatory effects of soil microorganisms upon Sclerotium rolfsii. Phytopath. 1955; 45(8) 417-20
Garrett S. Biology of root-infecting fungi. Cambridge University Press. 1956; 294 p. https://doi.org/10.1097/00010694-195607000-00011
Agrawal T, Kotasthane AS. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. Springerplus. 2012. https://doi.org/10.1186/2193-1801-1-73
Roberts WK, Selitrennikoff CP. Plant and bacterial chitinases differ in antifungal activity. Microbiology (N Y). 1988 Jan 1. https://doi.org/10.1099/00221287-134-1-169
Abdul-Baki AA, Anderson JD. Vigor determination in soybean seed by multiple criteria1. Crop Sci. 1973 Nov 1. https://doi.org/10.2135/cropsci1973.0011183X001300060013x
Jain A, Singh S, Kumar Sarma B, Bahadur Singh H. Microbial consortium-mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J Appl Microbiol]. 2012 Mar. https://doi.org/10.1111/j.1365-2672.2011.05220.x
Jensen B, Knudsen IMB, Madsen M, Jensen DF. Biopriming of infected carrot seed with an antagonist, Clonostachys rosea, selected for control of seedborne Alternaria spp. Phytopathology.2004. https://doi.org/10.1094/PHYTO.2004.94.6.551
Nirmaladevi D, Venkataramana M, Srivastava RK, Uppalapati SR, Gupta VK, Yli-Mattila T et al. Molecular phylogeny, pathogenicity and toxigenicity of Fusarium oxysporum f. sp. lycopersici. Scientific Reports. 2016 Feb 17 https://doi.org/10.1038/srep21367
Manganiello G, Sacco A, Ercolano MR, Vinale F, Lanzuise S, Pascale A et al. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite Harzianic acid. Front Microbiol. 2018 Aug 30;9(AUG):1966. https://doi.org/10.3389/fmicb.2018.01966
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973 Aug. https://doi.org/doi.org/10.1007/BF00018060
Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983 Jun;70(2):303-07. https://doi.org/10.1007/BF02374789
Tamburino R, Sannino L, Cafasso D, Cantarella C, Orrù L, Cardi T et al. Cultivated tomato (Solanum lycopersicumL.) suffered a severe cytoplasmic bottleneck during domestication: Implications from chloroplast genomes. Plants. 2020 Oct https://doi.org/10.3390/plants9111443
Singh VK, Singh AK, Kumar A. Disease management of tomato through PGPB: current trends and future perspective. 3 Biotech. 2017 Jul 20. https://doi.org/10.1007/s13205-017-0896-1
Adhikari TB, Gao A, Ingram T, Louws FJ. Pathogenomics characterization of an emerging fungal pathogen, Fusarium oxysporum f. sp. lycopersiciin greenhouse tomato productionsystems. Front Microbiol. 2020 Aug 27;11:1995. https://doi.org/10.3389/fmicb.2020.01995
Abdelghany MMA, Kurikawa M, Watanabe M, Matsui H, Yamamoto M, Ichinose Y et al. Surveillance of pathogenicity of Rhizoctonia solani Japanese isolates with varied anastomosis groups and subgroups on Arabidopsis thaliana. Life. 2022 Jan 1. https://doi.org/10.3390/life12010076
Heflish AA, Abdelkhalek A, Al-Askar AA, Behiry SI. Protective and curative effects of Trichoderma asperelloides ta41 on tomato root rot caused by Rhizoctonia solani Rs33. Agronomy. 2021 Jun 1;11(6). https://doi.org/10.3390/agronomy11061162
Larousse M, Rancurel C, Syska C, Palero F, Etienne C, Industri B et al. Tomato root microbiota and Phytophthora parasitica-associated disease. Microbiome. 2017;5(1). https://doi.org/10.1186/s40168-017-0273-7
Chaturvedi H, Singh B, Jajoo A, Prakash A. Shielding of photosynthetic apparatus by consortia of bacterial endophytes in tomato plants suffering from Fusarium wilt. Frontiers in Agronomy. 2022 May 18;4:33. https://doi.org/10.3389/fagro.2022.831731
Ajayi-Oyetunde OO, Bradley CA. Rhizoctonia solani: taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 2018 Jan 1. https://doi.org/10.1111/ppa.12733
Halifu S, Deng X, Song X, Song R, Liang X. Inhibitory mechanism of Trichoderma virens ZT05 on Rhizoctonia solani. Plants. 2020 Jul 19 . https://doi.org/10.3390/plants9070912
Zehra A, Meena M, Dubey MK, Aamir M, Upadhyay RS. Activation of defense response in tomato against Fusarium wilt disease triggered by Trichoderma harzianum supplemented with exogenous chemical inducers (SA and MeJA). Revista Brasil Botanica. 2017 Sep 1;40(3):651-64. https://doi.org/10.1007/s40415-017-0382-3
Ayub MA, Rehman MZU, Umar W, Farooqi ZUR, Sarfraz M, Ahmad HR et al. Role of glycine betaine in stress management in plants. Emerging Plant Growth Regulators in Agriculture: Roles in Stress Tolerance. https://doi.org/10.1016/B978-0-323-91005-7.00005-9
Aydi-Ben-Abdallah R, Jabnoun-Khiareddine H, Daami-Remadi M. Fusarium wilt biocontrol and tomato growth stimulation, using endophytic bacteria naturally associated with Solanum sodomaeum and S. bonariense plants. Egypt J Biol Pest Control. 2020 Dec 1. https://doi.org/10.1186/s41938-020-00313-1
Sood M, Kumar V, Rawal R. Seed biopriming a novel method to control seed borne diseases of crops. Biocontrol Agents and Secondary Metabolites. 2021 Jan 1; 181-223. https://doi.org/10.1016/B978-0-12-822919-4.00008-9
Abbas A, Mubeen M, Zheng H, Sohail MA, Shakeel Q, Solanki MK et al. Trichoderma spp. genes involved in the biocontrol activity against Rhizoctonia solani. Front Microbiol. 2022 May 25 .https://doi.org/10.3389/fmicb.2022.884469
Howell CR. Mechanisms employed by Trichodermaspecies in the biological control of plant diseases: The history and evolution of current concepts.2007 Feb 23. https://doi.org/10.1094/PDIS.2003.87.1.4
Harwoko H, Daletos G, Stuhldreier F, Lee J, Wesselborg S, Feldbrügge M et al. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat Prod Res. 2021. https://doi.org/10.1080/14786419.2019.1627348
Wang J, Song L, Gong X, Xu J, Li M. Functions of jasmonicacid in plant regulation and response to abiotic stress. Int J Mol Sci 2020. Feb 1. https://doi.org/10.3390/ijms21041446
Ameye M, Audenaert K, de Zutter N, Steppe K, van Meulebroek L, Vanhaecke L et al. Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium graminearum but boosts deoxynivalenol production. Plant Physiol. 2015 Apr 1;167(4):1671-84. https://doi.org/10.1104/pp.15.00107
Mei C, Qi M, Sheng G, Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression and host resistance to fungal infection. Mol Plant Microbe Interact. 2006 Oct. https://doi.org/10.1094/MPMI-19-1127
Zhao S, Li Y. Current understanding of the interplays between host hormones and plant viral infections. PLoSPathog. 2021 Feb 25;17(2). https://doi.org/10.1371/journal.ppat.1009242
Moosa A, Sahi S, Khan S, Horticulturae AMF, 2019 undefined. Salicylic acid and jasmonic acid can suppress green and blue moulds of citrus fruit and induce the activity of polyphenol oxidase and peroxidase. researchgate.net. 2019 Jun 1. https://doi.org/10.2478/fhort-2019-0014
Motallebi P, Niknam V, Ebrahimzadeh H, Hashemi M, Enferadi ST. Exogenous methyl jasmonatetreatment induces defenseresponse against Fusarium culmorum in wheat seedlings. J Plant Growth Regul. 2017 Mar 1;36(1):71-82. https://doi.org/10.1007/s00344-016-9620-3
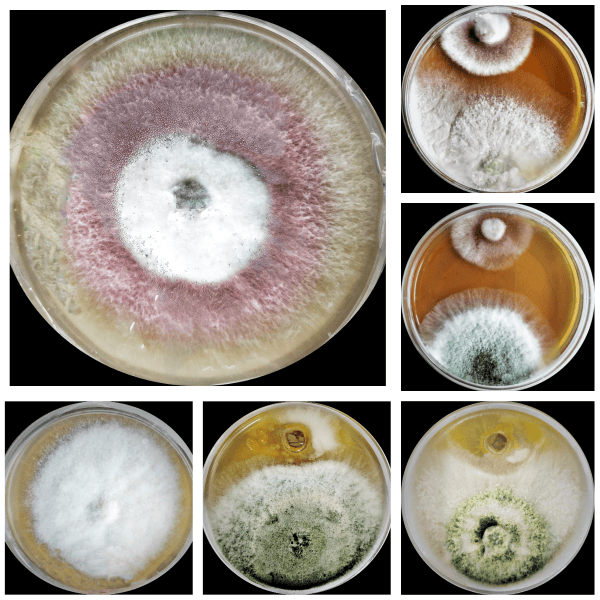
Downloads
Published
Versions
- 10-09-2023 (2)
- 07-08-2023 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Monika Sood, Sarvjeet Kukreja, Vipul Kumar

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









