Exploring the Impact of Resveratrol on Gynecological Cancer: Insights and Perspectives
DOI:
https://doi.org/10.14719/pst.2549Keywords:
Cervical cancer, Endometrioid cancer, Gynaecological malignancy, Resveratrol, Ovarian cancerAbstract
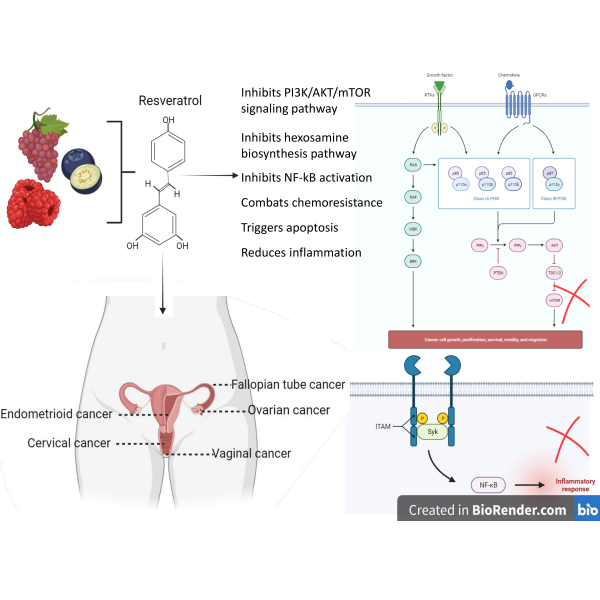
Gynecological cancers, a group of malignancies affecting the female reproductive system, are a significant cause of morbidity and mortality in women. Different types of gynecological cancers differ by distinct attributes, risk determinants, and therapeutic methodologies. So, depending on the type and stage of the cancer, a tailored combination of therapies is required for the treatment. However, it is shocking that the side effects of these therapeutic methods range from mild to severe. Hence, developing innovative therapeutic approaches to improve patient outcomes is imperative. Here's the juncture where the role of plant-derived compounds in curing gynecological cancers becomes evident. Various plant-derived compounds, including phytochemicals, polyphenols, alkaloids, and terpenoids for their cytotoxic, apoptotic, anti-angiogenic, and immunomodulatory properties, have been examined so far. Besides, certain phytocompounds can modulate hormonal-dependent gynecological cancers. Among the widely studied phytocompounds, RSV is the one that is extensively researched in vitro, in vivo and in-silico studies. In this context, this review article provides insights into the present-day knowledge about how RSV can potentially manage gynecological cancers. However, further research is needed to standardize their mode of action, optimal dosages, and potential interactions with conventional treatments. Rigorous clinical trials must validate their safety and efficacy profiles in different patient populations. As a result, a novel avenue for treating and preventing gynecological cancers could emerge by harnessing the multifaceted properties of phyto compounds, instilling new hope for patients and healthcare providers alike.
Downloads
References
Williams NF, Hauck YL, Bosco AM. Nurses' perceptions of providing psychosexual care for women experiencing gynaecological cancer. Eur J Oncol Nurs. 2017;30:35-42. https://doi.org/10.1016/j.ejon.2017.07.006
Asamoa-Afriyie CK. Papanicolaou Test Status Among Inner-City Adolescent Girls in Accra, Ghana (Doctoral dissertation, Walden University).
Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. Journal of the National Cancer Institute. 2018;110(4):354-61. https://doi.org/10.1093/jnci/djx214
Balogun O. Towards Global Equity in Women’s Cancer Care: An Assessment of Radiotherapy Utilization Among Uterine Cancer Patients in New York City and Breast Cancer Patients in Ife, Nigeria (Doctoral dissertation, Weill Medical College of Cornell University).
Sobstyl M, Brecht P, Sobstyl A, Mertowska P, Grywalska E. The role of microbiota in the immunopathogenesis of endometrial cancer. Int J Mol Sci. 2022;23(10):5756. https://doi.org/10.3390/ijms23105756
George SH, Donenberg T, Alexis C, DeGennaro V, Dyer H, Yin S, Ali J, Butler R, Chin SN, Curling D, Lowe D. Gene sequencing for pathogenic variants among adults with breast and ovarian cancer in the caribbean. JAMA Netw. Open. 2021;4(3):e210307-. https://doi:10.1001/jamanetworkopen.2021.0307
Lawrie TA, Bryant A, Cameron A, Gray E, Morrison J. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. CDSR. 2013(7). https://doi.org/10.1002/14651858.CD006910.pub2
Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat. Rev. Dis. 2016;2(1):1-22. https://doi.org/10.1038/nrdp.2016.61
Obermair A, Beale P, Scott CL, Beshay V, Kichenadasse G, Simcock B, Nicklin J, Lee YC, Cohen P, Meniawy T. Insights into ovarian cancer care: report from the ANZGOG Ovarian Cancer Webinar Series 2020. J Gynecol Oncol 2021;32(6). https://doi: 10.3802/jgo.2021.32.e95
NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. The Lancet. 2016;387(10026):1377-96. https://doi.org/10.1016/S0140-6736(16)30054-X
Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin. Oncol. Nurs. 2019 Apr 1 (Vol. 35, No. 2, pp. 151-156). https://doi.org/10.1016/j.soncn.2019.02.001
Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiology, Biomarkers and Prevention. 2009;18(1):132-9. https://doi.org/10.1158/1055-9965.EPI-08-0771
Rosalik K, Tarney C, Han J. Human papillomavirus vaccination. Viruses. 2021;13(6):1091. https://doi.org/10.3390/v13061091
Cozma AI, Martell K, Ravi A, Barnes E, Donovan E, Paudel M, Leung E, Taggar A. Relationship of urethral dose and genitourinary toxicity among patients receiving vaginal high dose rate interstitial brachytherapy. Clin. Oncol. 2021;33(12):773-9. https://doi.org/10.1016/j.clon.2021.05.006
Li J, Wu R, Qu X, Huang X, Li L, Lin Z, Zhang Z, Deng J, Liu R, Zhao X, Zhang S. Effectiveness and feasibility of self-sampling for human papillomavirus testing for internet-based cervical cancer screening. Front. Public Health. 2022;10:938272. https://doi.org/10.3389/fpubh.2022.938272
Dring JC, Forma A, Chilimoniuk Z, Dobosz M, Teresi?ski G, Buszewicz G, Flieger J, Cywka T, Januszewski J, Baj J. Essentiality of trace elements in pregnancy, fertility, and gynecologic cancers—a state-of-the-art review. Nutrients. 2021;14(1):185. https://doi.org/10.3390/nu14010185
Gebretsadik A, Bogale N, Dulla D. Descriptive epidemiology of gynaecological cancers in southern Ethiopia: retrospective cross-sectional review. BMJ open. 2022; 12(12):e062633. http://dx.doi.org/10.1136/bmjopen-2022-062633
Dehelean CA, Marcovici I, Soica C, Mioc M, Coricovac D, Iurciuc S, Cretu OM, Pinzaru I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules. 2021;26(4):1109. https://doi.org/10.3390/molecules26041109
Dehelean CA, Marcovici I, Soica C, Mioc M, Coricovac D, Iurciuc S, Cretu OM, Pinzaru I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules. 2021;26(4):1109. https://doi.org/10.3390/molecules26041109
Sun X, Fu P, Xie L, Chai S, Xu Q, Zeng L, Wang X, Jiang N, Sang M. Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int J Mol Med. 2021;47(1):335-45. https://doi.org/10.3892/ijmm.2020.4789
Kobylka P, Kucinska M, Kujawski J, Lazewski D, Wierzchowski M, Murias M. Resveratrol Analogues as Selective EstrogenSignaling Pathway Modulators: Structure–Activity Relationship. Molecules. 2022;27(20):6973. https://doi.org/10.3390/molecules27206973
Liang A, Huang LE, Liu H, He W, Lei X, Li M, Li S, Liang H, Chen G, Tang J, Chen F. Resveratrol improves follicular development of PCOS rats by regulating the glycolytic pathway. Mol Nutr Food Res. 2021;65(24):2100457. https://doi.org/10.1002/mnfr.202100457
Kim TH, Park JH, Woo JS. Resveratrol induces cell death through ROS?dependent downregulation of
Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol. Med. Rep. 2019;19(4):3353-60. https://doi.org/10.3892/mmr.2019.9962
Wahid M, Dar SA, Jawed A, Mandal RK, Akhter N, Khan S, Khan F, Jogaiah S, Rai AK, Rattan R. Microbes in gynecologic cancers: Causes or consequences and therapeutic potential. Semin Cancer Biol. 2022; 86:1179-89. https://doi.org/10.1016/j.semcancer.2021.07.013
Yu CK, Cutner A, Mould T, Olaitan A. Total laparoscopic hysterectomy as a primary surgical treatment for endometrial cancer in morbidly obese women. International Journal of Obstetrics and Gynaecology. 2005 ;112(1):115-7.https://doi.org/10.1111/j.1471-0528.2004.00335.x
Koutras A, Peteinaris A, Davakis S, Kalinterakis G, Tsilikis I, Garmpis N, Zotos PA, Chionis A, Schizas D, Karavokyros I, Thomakos N. Surgical versus conservative treatment for endometrial cancer in women of reproductive age: incidence of urinary tract symptoms. Anticancer Res. 2020;40(6):3065-9. https://doi.org/10.21873/anticanres.14287
Yaegashi N, Ito K, Niikura H. Lymphadenectomy for endometrial cancer: is paraaortic lymphadenectomy necessary. Int J Clin Oncol. 2007;12:176-80. https://doi.org/10.1007/s10147-006-0621-2
Magrina JF, Mutone NF, Weaver AL, Magtibay PM, Fowler RS, Cornella JL. Laparoscopic lymphadenectomy and vaginal or laparoscopic hysterectomy with bilateral salpingo-oophorectomy for endometrial cancer: morbidity and survival. American Journal of Obstetrics and Gynecology. 1999;181(2):376-81. https://doi.org/10.1016/S0002-9378(99)70565-X
Huang GS, Tymon-Rosario J, Santin AD. What role does adjuvant therapy play in the management of endometrial cancer?. Expert Opin Pharmacother. 2023;24(1):7-10. https://doi.org/10.1080/14656566.2022.2157207
Kuršvietien? L, Stanevi?ien? I, Mongirdien? A, Bernatonien? J. Multiplicity of effects and health benefits of resveratrol. Medicina. 2016;52(3):148-55. https://doi.org/10.1016/j.medici.2016.03.003
Zhong Z, Guo X, Zheng Y. Network Pharmacology-Based and Molecular Docking Analysis of Resveratrol's Pharmacological Effects on Type I Endometrial Cancer. Anticancer Agents Med Chem. 2022;22(10):1933-1944. https://doi.org/10.2174/1871520621666211015140455.
Xu X, Kong X, Liu T, Zhou L, Wu J, Fu J, Wang Y, Zhu M, Yao S, Ding Y, Ding L. Metastasis-associated protein 1, modulated by miR-30c, promotes endometrial cancer progression through AKT/mTOR/4E-BP1 pathway. Gynecol. Oncol. 2019 Jul 1;154(1):207-17. https://doi.org/10.1016/j.ygyno.2019.04.005
Yeung KT, Yang J. Epithelial–mesenchymal transition in tumor metastasis. Mol. Oncol. 2017 Jan;11(1):28-39. https://doi.org/10.1002/1878-0261.12017
Kong XY, Zhou H, Xu XF, Wu J, Zhou L, Zhu M, Wang Y, Yao S, Ding Y. Resveratrol inhibits the invasion and migration of endometrial cancer by reversing MTA1-ZEB2-induced epithelial-mesenchymal transition. Gynecol Oncol. 2020;159:38. https://doi.org/10.1016/j.ygyno.2020.06.080
Han G, Xia J, Gao J, Inagaki Y, Tang W, Kokudo N. Anti-tumor effects and cellular mechanisms of resveratrol. Drug Discov Ther. 2015;9(1):1-2. https://doi.org/10.5582/ddt.2015.01007
Wang M, Wu Y, He Y, Liu J, Chen Y, Huang J, Qi G, Li P. SIRT1 upregulation promotes epithelial-mesenchymal transition by inducing senescence escape in endometriosis. Sci. Rep. 2022;12(1):12302. https://doi.org/10.1038/s41598-022-16629-x
Lai H. A DNA methylation test from pap smears with abnormal uterine bleeding for endometrial cancer detection: A multicenter validating confirmatory study. Gynecol. Oncol. 2020;159:38. https://doi.org/10.1016/j.ygyno.2020.06.081
Srivastava S, Kumar P, Chaudhry V, Singh A. Detection of ovarian cyst in ultrasound images using fine-tuned VGG-16 deep learning network. SN comput. sci. 2020;1:1-8. https://doi.org/10.1007/s42979-020-0109-6
Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018;58(9):1428-47.https://doi.org/10.1080/10408398.2016.1263597
Zhong LX, Li H, Wu ML, Liu XY, Zhong MJ, Chen XY, Liu J, Zhang Y. Inhibition of STAT3 signaling as critical molecular event in resveratrol-suppressed ovarian cancer cells. J Ovarian Res. 2015;8:25. https://doi.org/10.1186/s13048-015-0152-4.
Zhang YK., Yang SF, Yang Y, Liu T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect. Agents Cancer. 2019;14:27. https://doi: 10.1186/s13027-019-0247-4.
Ferraresi A, Titone R, Follo C, Castiglioni A, Chiorino G, Dhanasekaran DN, Isidoro C. The protein restriction mimetic Resveratrol is an autophagy inducer stronger than amino acid starvation in ovarian cancer cells. Mol Carcinogen. 2017;56:2681–91. https://doi: 10.1002/mc.22711.
Gwak H, Kim S, Dhanasekaran DN, Song YS. Resveratrol triggers ER stress-mediated apoptosis by disrupting N-linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 2016;371:347–353. https://doi.org/10.1016/j.canlet.2015.11.032
Muhanmode Y, Wen MK, Maitinuri A, Shen G. Curcumin and resveratrol inhibit chemoresistance in cisplatin-resistant epithelial ovarian cancer cells via targeting P13K pathway. Hum Exp Toxicol. 2021;40:S861-S868. https://doi.org/10.1177/0960327122109592
Ferraresi A, Esposito A, Girone C, Vallino L, Salwa A, Ghezzi I, Thongchot S, Vidoni C, Dhanasekaran DN, Isidoro C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells. 2021;10(11):3213. https://doi.org/doi.org/10.3390/cells10113213
Yao S, Gao M, Wang Z, Wang W, Zhan L, Wei B. Upregulation of MicroRNA-34a Sensitizes Ovarian Cancer Cells to Resveratrol by Targeting Bcl-2. Yonsei Med J. 2021 Aug;62(8):691-701. https://doi: 10.3349/ymj.2021.62.8.691
Jalil AT, Karevskiy A. The cervical cancer (CC) epidemiology and human papillomavirus (HPV) in the middle east. Int. J. Environ. Eng. 2020;2(2):7-12.
D’Oria O, Corrado G, Laganà AS, Chiantera V, Vizza E, Giannini A. New advances in cervical cancer: from bench to bedside. International Journal of Environmental Research and Public Health. 2022;19(12):7094. https://doi.org/10.3390/ijerph19127094
Park KJ, Selinger CI, Alvarado-Cabrero I, Duggan MA, Kiyokawa T, Mills AM, Ordi J, Otis CN, Plante M, Stolnicu S, Talia KL. Dataset for the Reporting of Carcinoma of the Cervix: Recommendations From the International Collaboration on Cancer Reporting (ICCR). Int J Gynecol Pathol 2022;41:S64-89. https://doi.org/10.1097/PGP.0000000000000909
Kashyap N, Krishnan N, Kaur S, Ghai S. Risk factors of cervical cancer: a case-control study. Asia-Pac. J Oncol Nurs. 2019;6(3):308-14. https://doi.org/10.4103/apjon.apjon_73_18
Robles C, Bruni L, Acera A, Riera JC, Prats L, Poljak M, Mlakar J, Valen?ak AO, Eriksson T, Lehtinen M, Louvanto K. Determinants of human papillomavirus vaccine uptake by adult women attending cervical cancer screening in 9 European countries. Am J Prev Med. 2021;60(4):478-87. https://doi.org/10.1016/j.amepre.2020.08.032
Sakuragi N, Murakami G, Konno Y, Kaneuchi M, Watari H. Nerve-sparing radical hysterectomy in the precision surgery for cervical cancer. J Gynecol Oncol. 2020 May;31(3). https://doi.org/10.3802/jgo.2020.31.e49
Xu W, Xie S, Chen X, Pan S, Qian H, Zhu X. Effects of quercetin on the efficacy of various chemotherapeutic drugs in cervical cancer cells. Drug Des Devel Ther. 2021; 577-88. https://doi.org/10.2147/DDDT.S291865
Chargari C, Peignaux K, Escande A, Renard S, Lafond C, Petit A, Kee DL, Durdux C, Haie-Méder C. Radiotherapy of cervical cancer. Cancer Radiother. 2022;26(1-2):298-308. https://doi.org/10.1016/j.canrad.2021.11.009
Chen CP, Kung PT, Wang YH, Tsai WC. Effect of time interval from diagnosis to treatment for cervical cancer on survival: a nationwide cohort study. PLoS One. 2019;14(9):e0221946.https://doi.org/10.1371/journal.pone.0221946
Nadile M, Retsidou MI, Gioti K, Beloukas A, Tsiani E. Resveratrol against Cervical Cancer: Evidence from in vitro and in vivo Studies. Nutrients. 2022;14(24):5273. https://doi.org/10.3390/nu14245273
Yadav N, Parveen S, Banerjee M. Potential of nano-phytochemicals in cervical cancer therapy. Clin. Chim. Acta. 2020; 505:60-72. https://doi.org/10.1016/j.cca.2020.01.035
Komorowska D, Radzik T, Kalenik S, Rodacka A. Natural radiosensitizers in radiotherapy: cancer treatment by combining ionizing radiation with resveratrol. Int J Mol Sci. 2022;23(18):10627. https://doi.org/10.3390/ijms231810627
Liu Z, Li Y, She, G, Zheng X, Shao L, Wang P, Pang M, Xie S, Sun Y. Resveratrol Induces Cervical Cancer HeLa Cell Apoptosis through the Activation and Nuclear Translocation Promotion of FOXO3a. Pharmazie 2020; 75:250–254. https://doi.org/10.1691/ph.2020.0386
Ali D, Chen L, Kowal JM, Okla M, Manikandan M, AlShehri M, AlMana Y, AlObaidan R, AlOtaibi N, Hamam R, Alajez NM. Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells. Bone. 2020; 1;133:115252. https://doi.org/10.1016/j.bone.2020.115252
Sun X, Fu P, Xie L, Chai S, Xu Q, Zeng L, Wang X, Jiang N, Sang M. Resveratrol Inhibits the Progression of Cervical Cancer by Suppressing the Transcription and Expression of HPV E6 and E7 Genes. Int J Mol Med. 2021, 47, 335–45. https://doi.org/10.3892/ijmm.2020.4789
Adams TS, Rogers LJ, Cuello MA. Cancer of the vagina: 2021 update. Int. J. Gynecol. Obstet. 2021;155:19-27. https://doi.org/10.1002/ijgo.13867
Shrivastava SB, Agrawal G, Mittal M, Mishra P. Management of vaginal cancer. Rev Recent Clin Trials. 2015;10(4):289-97.
Hellman K, Silfversward C, Nilsson B, Hellstrom AC, Frankendal B, Pettersson F. Primary carcinoma of the vagina: Factors influencing the age at diagnosis. The radiumhemmet series 1956–96. Int J Gynecol Cancer. 2004;14:491–501. http://doi.org/10.1136/ijgc-00009577-200405000-00011
Adams TS, Cuello MA. Cancer of the vagina. Int J Gynecol. Obstet. 2018;143:14-21. https://doi.org/10.1002/ijgo.12610
De Hullu JA, Van der Zee AG. Surgery and radiotherapy in vulvar cancer. Crit. Rev. Oncol. Hemato. 2006;60(1):38-58. https://doi.org/10.1016/j.critrevonc.2006.02.008
Movva S, Rodriguez L, Arias?Pulido H, Verschraegen C. Novel chemotherapy approaches for cervical cancer. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2009;115(14):3166-80. https://doi.org/10.1002/cncr.24364
Kunos CA, Andrews SJ, Moore KN, Chon HS, Ivy SP. Randomized phase II trial of triapine-cisplatin-radiotherapy for locally advanced stage uterine cervix or vaginal cancers. Front Oncol. 2019;9:1067. https://doi.org/10.3389/fonc.2019.01067
Randall ME, Filiaci V, McMeekin DS, Von Gruenigen V, Huang H, Yashar CM, Mannel RS, Kim JW, Salani R, DiSilvestro PA, Burke JJ. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early-stage endometrial cancer. Clin Oncol. 2019; 37(21):1810. doi: 10.1200/JCO.18.01575
Chen S, Blaney L, Chen P, Deng S, Hopanna M, Bao Y, Yu G. Ozonation of the 5-fluorouracil anticancer drug and its prodrug capecitabine: Reaction kinetics, oxidation mechanisms, and residual toxicity. Front Environ Sci Eng. 2019;13:1-4. https://doi.org/10.1007/s11783-019-1143-2
Wang Y, Shen F, Zhou J, Fang Y, Qi Y, Chen Y. Overexpression of ARHI increases the sensitivity of cervical cancer cells to paclitaxel through inducing apoptosis and autophagy. Drug Dev Res. 2022;83(1):142-9. https://doi.org/10.1002/ddr.21852
Poltavets YI, Zhirnik AS, Zavarzina VV, Semochkina YP, Shuvatova VG, Krasheninnikova AA, Aleshin SV, Dronov DO, Vorontsov EA, Balabanyan VY, Posypanova GA. In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol. 2019;10(1):1-7. https://doi.org/10.1186/s12645-019-0048-x
Bagga R, Raghuvanshi P, Gopalan S, Das SK, Baweja R, Suri S, Malhotra D, Khare S, Talwar GP. A polyherbal vaginal pessary with spermicidal and antimicrobial action: evaluation of its safety. Trans R Soc Trop Med. Hyg. 2006;100(12):1164-7. https://doi.org/10.1016/j.trstmh.2006.01.008
Joglekar NS, Joshi SN, Navlakha SN, Katti UR, Mehendale SM. Acceptability of Praneem polyherbal vaginal tablet among HIV uninfected women & their male partners in Pune, India-Phase I study. IJMR. 2006;123(4):547.
Shukla S, Bharti AC, Hussain S, Mahata S, Hedau S, Kailash U, Kashyap V, Bhambhani S, Roy M, Batra S, Talwar GP. Elimination of high-risk human papillomavirus type HPV16 infection by ‘Praneem’polyherbal tablet in women with early cervical intraepithelial lesions. J Cancer Res Clin Oncol. 2009;135:1701-9. https://doi.org/10.1007/s00432-009-0617-1
Talwar, G, Raghuvanshi, P, Mishra, R, Banerjee, U, Rattan, A, Whaley, K.J, Achilles, S.L, Zeitlin, L, Barré-Sinoussi, F, David, A, et al. Polyherbal Formulations with Wide Spectrum Antimicrobial Activity Against Reproductive Tract Infections and Sexually Transmitted Pathogens. Am J Reprod Immunol. 2000; 43, 144–151. https://doi.org/10.1111/j.8755-8920.2000.430303.x
Saeed ME, Khalid HE, Thakur SK, Efferth T. Protein Expression Profiling and Virtual Drug Screening as an Approach for Individualized Therapy of Small Cell Vaginal Carcinoma. CGP. 2022;19(4):512-25. https://doi.org/10.21873/cgp.20337
Jøraholmen MW, Basnet P, Tostrup MJ, Moueffaq S, Škalko-Basnet N. Localized therapy of vaginal infections and inflammation: Liposomes-in-hydrogel delivery system for polyphenols. Pharm. 2019;11(2):53. https://doi.org/10.3390/pharmaceutics11020053
Mukherjee S, Debata PR, Hussaini R, Chatterjee K, Baidoo JN, Sampat S, Szerszen A, Navarra JP, Fata J, Severinova E, Banerjee P. Unique synergistic formulation of curcumin, epicatechin gallate and resveratrol, tricurin, suppresses HPV E6, eliminates HPV+ cancer cells, and inhibits tumor progression. Oncotarget. 2017;8(37):60904. https://doi.org/ 10.18632/oncotarget.16648
Einbond LS, Zhou J, Wu HA, Mbazor E, Song G, Balick M, DeVoti JA, Redenti S, Castellanos MR. A novel cancer preventative botanical mixture, TriCurin, inhibits viral transcripts and the growth of W12 cervical cells harbouring extrachromosomal or integrated HPV16 DNA. Br. J. Cancer. 2021;124(5):901-13. https://doi.org/10.1038/s41416-020-01170-3
Piao L, Mukherjee S, Chang Q, Xie X, Li H, Castellanos MR, Banerjee P, Iqbal H, Ivancic R, Wang X, Teknos TN. TriCurin, a novel formulation of curcumin, epicatechin gallate, and resveratrol, inhibits the tumorigenicity of human papillomavirus-positive head and neck squamous cell carcinoma. Oncotarget 2017;8(36):60025. https://doi.org/ 10.18632/oncotarget.10620
Ledford LR, Lockwood S. Scope and epidemiology of gynecologic cancers: an overview. InSeminars in oncology nursing 2019; 35:147-50. https://doi.org/10.1016/j.soncn.2019.03.002
Thurmond AS. Fallopian tube catheterization. Semin. Interv. Radiol. 2008;25:425-431. https://doi.org/10.1055/s-0033-1359732
Brüssow KP, Ratky J, Rodriguez?Martinez H. Fertilization and early embryonic development in the porcine fallopian tube. Reprod. Domest. Anim. 2008;43:245-51. https://doi.org/10.1111/j.1439-0531.2008.01169.x
Hundal J, Lopetegui-Lia N, Rabitaille W. Fallopian tube cancer–challenging to diagnose but not as infrequent as originally thought. Journal of community hospital internal medicine perpectives 2021;11(3):393-6.https://doi.org/10.1080/20009666.2021.1893889
Yucer N, Ahdoot R, Workman MJ, Laperle AH, Recouvreux MS, Kurowski K, Naboulsi DJ, Liang V, Qu Y, Plummer JT, Gayther SA. Human iPSC-derived fallopian tube organoids with BRCA1 mutation recapitulate early-stage carcinogenesis. Cell Rep. 2021;37(13). https://doi.org/10.1016/j.celrep.2021.110146
Zheng W, Sung CJ, Cao P, et al. Early occurrence and prognostic significance of p53 alteration in primary carcinoma of the fallopian tube. Gynecol Oncol. 1997;64:38–48. https://doi.org/10.1006/gyno.1996.4519
Aziz S, Kuperstein G, Rosen B, et al. A genetic epidemiological study of carcinoma of the fallopian tube. Gynecol Oncol. 2001;80:341–345. https://doi.org/10.1006/gyno.2000.6095
Chang YH, Chu TY, Ding DC. Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation. J Biomed Sci. 2020;27:1-2. https://doi.org/10.1186/s12929-019-0602-1
Bohiltea RE, Bacalbasa N, Balescu I, Mitran M, Georgescu TA, Grigoriu C, Gheorghe CM, Vladareanu IT, Berceanu10 C. Abnormal uterine bleeding: Terminology, FIGO classification and management. RMJ. 2021;68(6):49. https://doi.org/10.37897/RMJ.2021.S6.8
Hunt R, Quigley E, Abbas Z, Eliakim A, Emmanuel A, Goh KL, Guarner F, Katelaris P, Smout A, Umar M, Whorwell P. Coping with common gastrointestinal symptoms in the community: a global perspective on heartburn, constipation, bloating, and abdominal pain/discomfort May 2013. J. Clin. Gastroenterol. 2014;48(7):567-78. https://doi.org/ 10.1097/MCG.0000000000000141
Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2015;131:S111-22. https://doi.org/10.1002/ijgo.12614
Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynecol Obstet. 2021;155:61-85. https://doi.org/10.1002/ijgo.13878
Rizzuto I, Oehler MK, Lalondrelle S. Sexual and psychosexual consequences of treatment for gynaecological cancers. Clin Oncol 2021;33(9):602-7. https://doi.org/10.1016/j.clon.2021.07.003
Revzin MV, Moshiri M, Katz DS, Pellerito JS, Mankowski Gettle L, Menias CO. Imaging evaluation of fallopian tubes and related disease: a primer for radiologists. Radiographics. 2020;40(5):1473-501. https://doi.org/10.1148/rg.2020200051
Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12:1-9. https://doi.org/10.1186/s13048-019-0503-7
Ajithkumar TV, Minimole AL, John MM, Ashokkumar OS. Primary fallopian tube carcinoma. Obstet Gynecol Surv. 200560(4):247-52. https://doi.org/ 10.1097/01.ogx.0000158506.23663.79
Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, Iwabe T, Terakawa N. Tumor necrosis factor-?-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-?B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab. 2003;88(2):730-5. https://doi.org/10.1210/jc.2002-020666
Harris T, Vlass AM. Can herbal medicines improve cellular immunity patterns in endometriosis. Med. Aromat. Plants. 2015;4(2). http:// doi.org/10.4172/2167-0412.1000184
Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. Resveratrol blocks interleukin-1?–induced activation of the nuclear transcription factor NF-?B, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102(3):987-95. https://doi.org/10.1182/blood-2002-11-3550
Downloads
Published
Versions
- 23-10-2023 (2)
- 15-10-2023 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Unni K Revathi, Pavithra Amrisa, Kumar G Aparna, K S Santhy

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









