Effect of salinity on DNA methylation and antioxidant phenolic compounds of wild watercress (Rorippa nasturtium aquaticum)
DOI:
https://doi.org/10.14719/pst.2577Keywords:
watercress, methylation, metabolites, stress, phenolics, antioxidantAbstract
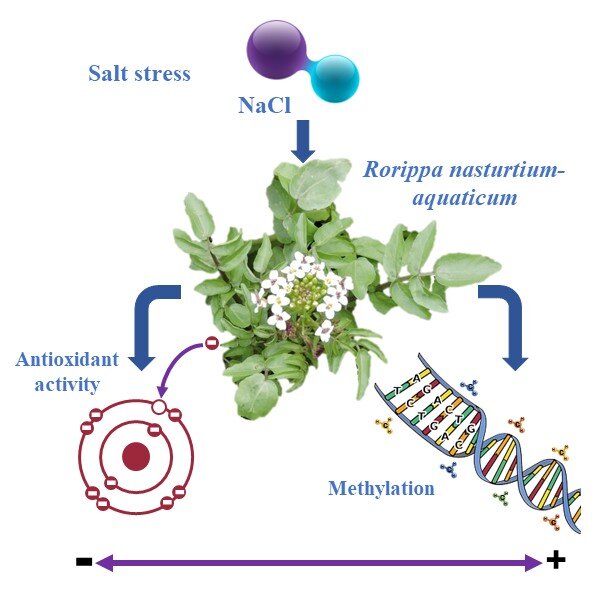
Epigenetic changes are involved in plant responses to stress. Cytosine methylation is one of the most important epigenetic changes, regulating gene expression. In this paper, the MSAP (methylation-sensitive amplification polymorphism) method was used to find out how the watercress (Rorippa nasturtium aquaticum) genome changed in response to 0, 60, 80, and 100 mM NaCl and how that affected phenylalanine ammonium lyase (PAL) activity, phenolic content, and antioxidant capacity. The results showed an inverse correlation between methylation levels and PAL activity and the contents of total phenolics and flavonoids, indicating salt stress-induced reprogramming of the methylation pattern of watercress, which has a negative effect on the synthesis of phenolics. The results revealed a significant decrease in phenolic contents and antioxidant activity under low and moderate salinity compared to control and an increase under strong salinity compared to moderate salinity. The findings of this study contribute to our understanding of the reprogramming of DNA methylation under salinity and its effect on watercress phenolic metabolism.
Downloads
References
Rejeb IB, Pastor V, Mauch-Mani B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants. 2014;3:458-75. https://doi.org/10.3390/plants3040458
You J, Chan Z. ROS regulation during abiotic stress responses in crop plants. Front Plant Sci. 2015;6:1092. https://doi.org/10.3389/fpls.2015.01092
Dumanovic J, Nepovimova E, Natic M, Kuca K, Jacevic V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front Plant Sci. 2020;11:552969. https://doi.org/10.3389/fpls.2020.552969
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9:681. https://doi.org/10.3390/antiox9080681
Sapna H, Ashwini N, Ramesh S, Nataraja K. Assessment of DNA methylation pattern under drought stress using methylation-sensitive randomly amplified polymorphism analysis in rice. Plant Genet Resour: Characterization and Utilization. 2020;18:222-30. https://doi:10.1017/S1479262120000234
Sicilia A, Scialò E, Puglisi I, Lo Piero AR. Anthocyanin biosynthesis and DNA methylation dynamics in sweet orange fruit [Citrus sinensis L.(Osbeck)] under cold stress. J Agric Food Chem. 2020;68:7024-31. https://doi:10.1021/acs.jafc.0c02360
Lee HM, Park JS, Shin YH, Park YD. Alterations in DNA methylation patterns in regenerated Chinese cabbage (Brassica rapa ssp. pekinensis) plants derived from tissue culture. Hortic Environ Biotechnol. 2021;62:605-18. https://doi:10.1007/s13580-020-00310-1
Gallusci P, Dai Z, Génard M, Gauffretau A, Leblanc-Fournier N, Richard-Molard C et al. Epigenetics for plant improvement: Current knowledge and modeling avenues. Trends Plant Sci. 2017;22(7):610-23. http://dx.doi.org/10.1016/j.tplants.2017.04.009
Shahrajabian MH, Sun W, Cheng Q. DNA methylation as the most important content of epigenetics in traditional Chinese herbal medicine. J Med Plant Res. 2019;13:357-69. https://doi:10.5897/JMPR2019.6803
González-Benito ME, Ibáñez MÁ, Pirredda M, Mira S, Martín C. Application of the MSAP technique to evaluate epigenetic changes in plant conservation. Int J Mol Sci. 2020;21:7459. https://doi:10.3390/ijms21207459
Lira-Medeiros CF, Parisod C, Fernandes RA, Mata CS, Cardoso MA, Ferreira PC. Epigenetic variation in mangrove plants occurring in contrasting natural environment. PLoS One. 2010;26:5(4):e10326. https://doi:10.1371/journal.pone.0010326
Kokhdan EP, Khodabandehloo H, Ghahremani H, Doustimotlagh AH. A narrative review on therapeutic potentials of watercress in human disorders. Evid Based Complement Alternat Med. 2021;7:5516450. https://doi:10.1155/2021/5516450
González-Elizondo M, López-Enríquez IL, González-Elizondo MS, Tena-Flores JA. Plantas medicinales del estado de durango y zonas aledañas. Instituto Politécnico Nacional–PROSIMA, México; 2004.
Timpano AJ, Zipper CE, Soucek DJ, Schoenholtz SH. Seasonal pattern of anthropogenic salinization in temperate forested headwater streams. Water Res. 2018;15:133:8-18. https://doi:10.1016/j.watres.2018.01.012
Ladwig R, Rock LA, Dugan HA. Impact of salinization on lake stratification and spring mixing. Limnol Oceanogr Lett. 2023;8:93-102. https://doi.org/10.1002/lol2.10215
Bartwal A, Mall R, Lohani P, Guru SK, Arora S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul. 2013;32:216-32. https://doi.org/10.1007/s00344-012-9272-x
Naikoo MI, Dar MI, Raghib F, Jaleel H, Ahmad B, Raina A et al. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In: Plant Signaling Molecules (Ed: Raina A). Academia. 2019;p. 157-68. doi: https://doi.org/10.1016/B978-0-12-816451-8.00009-5
Bharti P, Mahajan M, Vishwakarma AK, Bhardwaj J, Yadav SK. AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco. J Exp Bot. 2015;66:5959-69. https://doi:10.1093/jxb/erv304
Gutiérrez-Velázquez MV, Almaraz-Abarca N, Herrera-Arrieta Y, Ávila-Reyes JA, González-Valdez LS, Torres-Ricario R et al. Comparison of the phenolic contents and epigenetic and genetic variability of wild and cultivated watercress (Rorippa nasturtium var. aquaticum L.). Electron J Biotechnol. 2018;34:9-16. https://doi:10.1016/j.ejbt.2018.04.005
Skotti E, Anastasaki E, Kanellou G, Polissiou M, Tarantilis PA. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind Crops Prod. 2014;53:46-54. https://doi:10.1016/j.indcrop.2013.12.013
Ordoñez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006;97:452-58. https://doi:10.1016/j.foodchem.2005.05.024
Anand T, Chandrasekaran A, Kuttalam S, Raguchander T, Prakasam V, Samiyappan R. Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J Plant Interact. 2007;2:233-44. https://doi:10.1080/17429140701708985
Medina-Medrano JR, Almaraz-Abarca N, González-Elizondo MS, Uribe-Soto JN, González-Valdez LS, Herrera-Arrieta Y. Phenolic constituents and antioxidant properties of five wild species of Physalis (Solanaceae). Bot Stud. 2015;56:24-37. https://doi:10.1186/s40529-015-0101-y
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;6(9-10):1231-37. https://doi:10.1016/s0891-5849(98)00315-3
Chavan RR, Bhinge SD, Bhutkar MA, Randive DS, Wadkar GH, Todkar SS et al. Characterization, antioxidant, antimicrobial and cytotoxic activities of green synthesized silver and iron nanoparticles using alcoholic Blumea eriantha DC plant extract. Mater Today Commun. 2020;24:101320. https://doi.org/10.1016/j.mtcomm.2020.101320
Bhau BS, Gogoi G, Baruah D, Ahmed R, Hazarika G, Borah B et al. Development of an effective and efficient DNA isolation method for Cinnamomum species. Food Chem. 2015;188:264-70. https://doi:10.1016/j.foodchem.2015.05.004
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2021. https://www.R-project.org/
Salmon A, Clotault J, Jenczewski E, Chable V, Manzanares-Dauleux MJ. Brassica oleracea displays a high level of DNA methylation polymorphism. Plant Sci. 2008;174:61-70. https://doi:10.1016/j.plantsci.2007.09.012
Yuan G, Wang X, Guo R, Wang Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010;121:1014-19. https://doi:10.1016/j.foodchem.2010.01.040
Valifard M, Mohsenzadeh S, Kholdebarin B, Rowshan V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S Afr J Bot. 2014;93:92-97. https://doi:10.1016/j.sajb.2014.04.002
Zhou Y, Tang N, Huang L, Zhao Y, Tang X, Wang K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density and volatile exudates of Schizonepeta tenuifolia Briq. Int J Mol Sci. 2018;19:252. https://doi:10.3390/ijms19010252
Telesinski A, Nowak J, Smolik B, Dubowska A, Skrzypiec N. Effect of soil salinity on activity of antioxidant enzymes and content of ascorbic acid and phenols in bean (Phaseolus vulgaris L.) plants. J Elem. 2008;13:401-09.
Falcinelli B, Benincasa P, Calzuola I, Gigliarelli L, Lutts S, Marsili V. Phenolic content and antioxidant activity in raw and denatured aqueous extracts from sprouts and wheatgrass of einkorn and emmer obtained under salinity. Molecules. 2017;2:22(12):2132. https://doi:10.3390/molecules22122132
Monreal-García HM, Almaraz-Abarca N, Ávila-Reyes JA, Torres-Ricario R, González-Elizondo MS, Herrera-Arrieta Y. Phytochemical variation among populations of Fouquieria splendens Engelm. (Fouquieriaceae). Bot Sci. 2019;97(3):398-412. https://doi.org/10.17129/botsci.2191
Tuteja N, Gill SS, Tuteja R. Plant responses to abiotic stresses: Shedding light on salt, drought, cold and heavy metal stress. In: Tuteja N, Gill SS, Tuteja R, editors. Omics and Plant Abiotic Stress Tolerance. e-book, Bentham Science Publishers; 2011. p.39–61. https://doi:10.2174/97816080505811110101
Aymen S, Morena G, Vincenzo L, Laura P, Lorenza B, Abderrazak S et al. Salt tolerance of the halophyte Limonium delicatulum is more associated with antioxidant enzyme activities than phenolic compounds. Funct Plant Biol. 2016;43:607-19. https://doi:10.1071/FP15284
Hernández-Pacheco CE, Almaraz-Abarca N, Rojas-López M, Torres-Ricario R, Ávila-Reyes JA, González-Valdez LS et al. Salinity generates varying chemical and biochemical responses in Physalis ixocarpa (Solanaceae) during different times of exposure. Electron JBiotech. 2022;59:25-35. https://doi.org/10.1016/j.ejbt.2022.06.002
Farhadi N, Ghassemi-Golezani K. Physiological changes of Mentha pulegium in response to exogenous salicylic acid under salinity. Sci Hortic. 2020;267(1-8):109325. https://doi:10.1016/j.scienta.2020.109325
Gao S, Ouyang C, Wang S, Xu Y, Tang L, Chen F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant Soil Environ. 2008;54:374-81. https://doi:doi.org/10.17221/410-PSE
Gholizadeh A, Kohnehrouz BB. Activation of phenylalanine ammonia lyase as a key component of the antioxidative system of salt-challenged maize leaves. Brazilian J Plant Physiol. 2010;22:217-23. https://doi:10.1590/S1677-04202010000400001
Bahramikia S, Yazdanparast R. Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. J Acupunct Meridian Stud. 2010;3:283-90. https://doi:10.1016/S2005-2901(10)60049-0
Rebey IB, Bourgou S, Rahali FZ, Msaada K, Ksouri R, Marzouk B. Relation between salt tolerance and biochemical changes in cumin (Cuminum cyminum L.) seeds. J Food Drug Anal. 2017;25:391-402. https://doi:10.1016/j.jfda.2016.10.001
Djenidi H, Khennouf S, Bouaziz A. Antioxidant activity and phenolic content of commonly consumed fruits and vegetables in Algeria. Prog Nutr. 2020;22:224-35. https://doi:10.23751/pn.v22i1.7701
Xu X, Li T, Li Y, Wang H. Variation of DNA cytosine methylation patterns among parent lines and reciprocal hybrids in hot pepper. Chem Eng Trans. 2015;46:1345-50. https://doi:10.3303/CET1546225
Tiwari JK, Saurabh S, Chandel P, Singh BP, Bhardwaj V. Analysis of genetic and epigenetic variation in in vitro propagated potato somatic hybrid by AFLP and MSAP marker. Electron J Biotech. 2013;16:6. https://doi:10.2225/vol16-issue6-fulltext-9
Sun M, Yang Z, Liu L, Duan L. DNA methylation in plant responses and adaption to abiotic stresses. Int J Mol Sci. 2022;23:6910. https://doi:10.3390/ijms23136910
Shan X, Wang X, Yang G, Wu Y, Su S, Li S, Liu H, Yuan Y. Analysis of the DNA methylation of maize (Zea mays L.) in response to cold stress based on methylation-sensitive amplified polymorphisms. J Plant Biol. 2013;56:32-38. https://doi:10.1007/s12374-012-0251-3
Akhter Z, Bi Z, Ali K, Sun C, Fiaz S, Haider FU, Bai J. In response to abiotic stress, DNA methylation confers epigenetic changes in plants. Plants. 2021;10:1096. https://doi:10.3390/plants10061096
Shi W, Hu X, Chen X, Ou X, Yang J, Geng Y. Increased population epigenetic diversity of the clonal invasive species Alternanthera philoxeroides in response to salinity stress. Genes Genet Syst. 2018;93:259-69. https://doi:10.1266/ggs.18-00039
Shahrajabian MH, Sun W, Cheng Q. DNA methylation as the most important content of epigenetics in traditional Chinese herbal medicine. J Med Plants Res. 2019;13:357-69. https://doi:10.5897/JMPR2019.6803
Downloads
Published
Versions
- 02-01-2024 (2)
- 20-10-2023 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Marcela Verónica Gutiérrez-Velázquez, Norma Almaraz-Abarca, José Antonio Ávila-Reyes, Eli Amanda Delgado-Alvarado, Laura Silvia González-Valdez, Rene Torres-Ricario, Hugo Manuel Monreal-García, Dante Yamid Rojas-Barboza, Andrés Vasavilbazo-Saucedo

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









