Double phase culture system mediated enhanced protocol for shoot proliferation of Vanilla andamanica Rolfe. - an endemic wild relative of commercial Vanilla from the Andaman and Nicobar Islands
DOI:
https://doi.org/10.14719/pst.2475Keywords:
Vanilla andamanica, Endemic, Double phase culture, Liquid medium, Micropropagation, Shoot proliferationAbstract
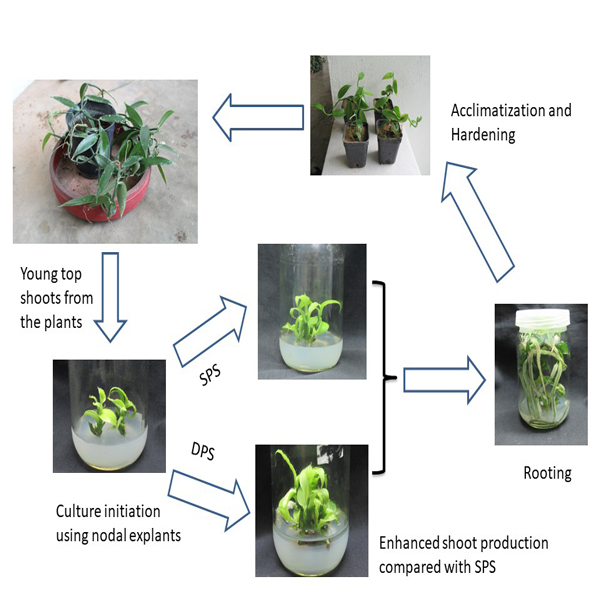
A liquid overlay culture system in micropropagation improved shoot proliferation in many species. In the present investigation, we have developed an enhanced shoot proliferation protocol using the double phase culture system (DPS) for Vanilla andamanica, an endemic species of the Andaman and Nicobar Islands recognized under the vulnerable category by the IUCN. In vitro generated nodal explants were used for shoot proliferation experiments and were tested in Murashige and Skoog medium augmented with various cytokinins (BAP, KIN, 2iP) and auxins (IBA, NAA, IAA) to produce a maximum of 3.24 ± 0.08 shoots per explant in 5 µM BAP and 5.23±0.67 roots per shoot in basal MS medium. A remarkable increase in shoot regeneration was observed when the nodal explant was cultured on DPS system with optimum cytokinin (5 µM BAP). On evaluation of the influence of DPS and conventional single-phase systems (SPS), (solid medium) exhibits an improved multiplication rate on a DPS with optimal BAP (5µM) with an average of 8.66 ± 0.17 shoots per explant, which represents a 2-fold increase over the rate of SPS + 5 µM BAP. The plantlets were rooted at 100% frequency in half-strength MS medium devoid of auxins and acclimatized with 100% success. A double-phase mediated enhanced shoot proliferating procedure could be employed for large scale multiplication and commercial breeding trials for better results with regard to V. andamanica.
Downloads
References
Rodolphe G, Séverine B, Michel G, Pascale B. Biodiversity and evolution in the Vanilla genus. The dynamical processes of biodiversity-case studies of evolution and spatial distribution. 2011 Dec;2:1-27. https://doi.org/10.5772/24567
Bory S, Grisoni M, Duval MF, Besse P. Biodiversity and preservation of vanilla: Present state of knowledge. 2008;551-71. https://doi.org/10.1007/s10722-007-9260-3
Verma PC, Chakrabarty D, Jena SN, Mishra DK, Singh PK, Sawant SV, Tuli R. The extent of genetic diversity among Vanilla species: Comparative results for RAPD and ISSR. Industrial Crops and Products. 2009 Mar 1;29(2-3):581-89. https://doi.org/10.1016/j.indcrop.2008.11.006
Lubinsky P. Conservation of wild vanilla. In First International Congress on the Future of the Vanilla Business. Princeton, NJ, USA. 2003 Nov 11; pp. 11-12.
Minoo DI, Jayakumar VN, Veena SS, Vimala J, Basha A, Saji KV, Nirmal Babu K, Peter KV. Genetic variations and interrelationships in Vanilla planifolia and few related species as expressed by RAPD polymorphism. Genetic Resources and Crop Evolution. 2008 May;55:459-70. https://doi.org/10.1007/s10722-007-9252-3
Divakaran M, Pillai GS, Babu KN, Peter KV. Isolation and fusion of protoplasts in Vanilla species. Current Science. 2008 Jan; 10:115-20.
Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962 Jul;15(3):473-97. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Abebe Z, Mengesha A, Teressa A, Tefera W. Efficient in vitro multiplication protocol for Vanilla planifolia using nodal explants in Ethiopia. African Journal of Biotechnology. 2009;8 (24). https://doi.org/10.4314/AJB.V8I24.68674
de Oliveira SO, Sayd RM, Balzon TA, Scherwinski-Pereira JE. A new procedure for in vitro propagation of vanilla (Vanilla planifolia) using a double-phase culture system. Scientia Horticulturae. 2013 Sep 24;161:204-09.https://doi.org/10.1016/j.scienta.2013.06.039
Divakaran M, Babu KN, Peter KV. Conservation of Vanilla species, in vitro. Scientia Horticulturae. 2006 Oct 9;110(2):175 -80.https://doi.org/10.1016/j.scienta.2006.07.003
Sota V, Benelli C, Çuko B, Kongjika E. Effect of a double phase culture system and activated charcoal on in vitro propagation of Malus sylvestris (L.) Mill. Advances in Horticultural Science. 2022 Oct 1;35(4):361.https://doi.org/10.36253/ahsc-11825
Feuser S, Meler K, Daquinta M, Guerra MP, Nodari RO. Genotypic fidelity of micropropagated pineapple (Ananas comosus) plantlets assessed by isozyme and RAPD markers. Plant Cell, Tissue and Organ Culture. 2003 Mar;72:221-27. https://doi.org/10.1023/A:1022321405683
Scherwinski-Pereira JE, Lima ED, da Silva TL, Mesquita AG, Maciel SD, da Silva Costa FH. Double-phase culture system for large scale production of pineapple. Plant Cell, Tissue and Organ Culture. 2012 May;109:263-69. https://doi.org/10.1007/s11240-011-0091-8
Roy AR, Sajeev S, Pattanayak A, Deka BC. TDZ induced micropropagation in Cymbidium giganteum Wall. Ex Lindl. and assessment of genetic variation in the regenerated plants. Plant Growth Regulation. 2012;68:435-45. https://doi.org/10.1007/s10725-012-9732-0
Moraes LK, Felisbino C, Crestani L, Silva AL. In vitro establishment and multiplication of Pyrus calleryana D-6 on double-phase culture system. RevistaBrasileira de Fruticultura. 2004;26:403-05. https://doi.org/10.1590/S0100-29452004000300008
Pereira JE, Fortes GR. Protocol for the production of potato propagating material in liquid medium. Brazilian Agricultural Research. 2003;38:1035-43. https://doi.org/10.1590/S0100-204X2003000900003
López D C, Suárez IE. In vitro arrow cane (Gynerium sagitatum Aubl.) multiplication in double phase medium. Revista de CienciasAgrícolas. 2018 Dec;35(2):5-13. https://doi.org/10.22267/rcia.183502.86
Sandhyarani N, Kishor R, Sharma GJ. Clonal propagation of triploid Acorus calamus Linn. using dual-phase culture system. Journal of Crop Science and Biotechnology. 2011 Sep;14:213-17. https://doi.org/10.1007/s12892-011-0028-0
Kalimuthu K, Senthilkumar R, Murugalatha N. Regeneration and mass multiplication of Vanilla planifolia Andr.–A tropical orchid. Current Science. 2006 Nov; 25:1401-03.
Gurel E, Yucesan B, Aglic E, Gurel S, Verma SK, Sokmen M, Sokmen A. Regeneration and cardiotonic glycoside production in Digitalis davisiana Heywood (Alanya foxglove). Plant Cell, Tissue and Organ Culture (PCTOC). 2011 Feb;104:217-25.
Downloads
Published
Versions
- 02-10-2023 (2)
- 28-07-2023 (1)
How to Cite
Issue
Section
License
Copyright (c) 2022 Shaina Jerald, A Gangaprasad, Sam P Mathew

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright and Licence details of published articles
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Open Access Policy
Plant Science Today is an open access journal. There is no registration required to read any article. All published articles are distributed under the terms of the Creative Commons Attribution License (CC Attribution 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/). Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).









